Background: Acute myeloid leukemia (AML) is a clinically and molecularly heterogeneous disorder. Bone marrow (BM) constitutes the home niche for leukemia cells in AML. Emerging data indicates that the BM microenvironment becomes immunosuppressive and plays a crucial role in cancer development and progression. Regulatory T cells (Treg), tumor associated macrophages (TAM) and myeloid-derived suppressor cells (MDSC) all contribute to immunologically permissive microenvironment for cancer cells. Based on phenotypical characteristics, MDSC can be further subdivided into granulocytic MDSC (G-MDSC, polymorphonuclear MDSC) and monocytic MDSC (M-MDSC). Although increasing evidence suggests that the immune system impacts the pathogenesis and prognosis in AML patients, only limited data has been published to comprehensively describe the immunological composition of AML BM microenvironment.
Methods: In this study, we aimed to perform comprehensive characterization of the immune cells in the BM of patients with AML. Using MultiOmyx hyperplexed immunofluorescence (IF) assay and proprietary imaging analysis, we studied BM tissues of 20 AML patients and 6 normal controls with a total of 13 markers essential in cancer immunology. The normal and AML BM FFPE sections were stained with CD34, Arginase1, CD11b, CD14, CD15, CD33, CD68, CD163, HLA-DR, CD3, CD4, CD8 and FOXP3.
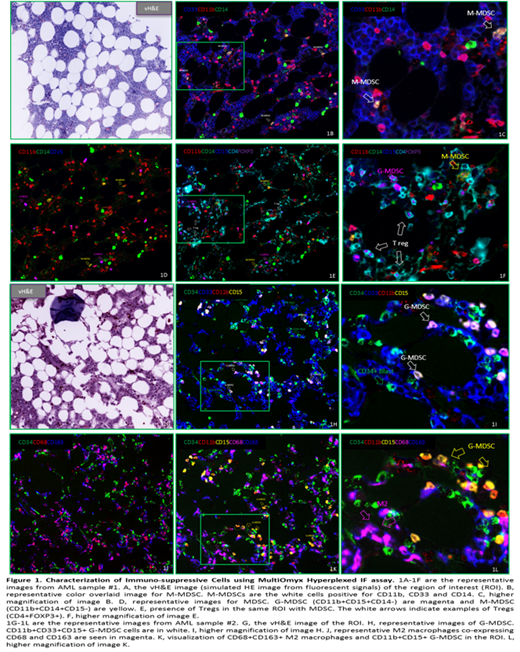
Results: Overall, MultiOmyx 13-plex panel staining results revealed an immune suppression-skewed immune profile in AML BM in this study. We observed that both M-MDSC and G-MDSC accumulated within the TME in AML BM samples, with higher frequency of G-MDSCs over M- MDSCs. The data also revealed an abundant M2 macrophages present in the TME of the AML samples. The detection of both MDSCs and M2 macrophages in these samples supports the hypothesis that these cells contribute to the establishment of an immunosuppressive TME. Using the MultiOmyx proprietary algorithm, which takes into account the staining patterns, we quantified the counts and density of different immune cells in both AML patient and normal BM samples. There was a significantly higher frequency of M2 TAM in AML than normal BM. Increased M-MDSC to G-MDSC ratio was also noted in patients with AML. Further, the spatial distance from the different subsets of immunosuppressive cells to CD34+ blasts was measured in AML samples using nearest neighbor analysis. The data indicated that G-MDSC were spatially closer to CD34+ blasts in AML than M-MDSC.
Conclusions: The direct assessment of immune phenotypes and their spatial relationship by MultiOmyx IF assay provides essential information in understanding the immune landscape in AML BM. Together, our data suggests that AML blasts may directly recruit immunosuppressive Tregs, MDSC and TAM and this may be one of the escape strategies. The potential for eradicating AML lies in rational combinations of immunotherapies with strategies of the induction of anti-tumor immunity and the elimination or reprogramming of the immunosuppressive TME.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal