Introduction: Marginal zone lymphoma (MZL) is an indolent B-cell malignancy comprising >10% of non-Hodgkin lymphomas. The Bruton's tyrosine kinase inhibitor ibrutinib was the first systemic therapy approved by the US Food and Drug Administration (FDA) for the treatment of patients (pts) with relapsed or refractory MZL, based on a Phase II study reporting an objective response rate (ORR) of 48% (3% complete responses [CR]) and a median progression-free survival (PFS) of 14.2 months (mo) in 60 pts (Noy A et al. Blood 2017). Copanlisib is a pan-class I phosphatidylinositol 3-kinase inhibitor with potent activity against the α and δ isoforms, approved for the treatment of relapsed follicular lymphoma in pts who have received ≥2 prior systemic therapies. In May 2019, the FDA granted Breakthrough Therapy designation for copanlisib for adults with MZL who have received ≥2 prior systemic therapies. We report results from 23 heavily pretreated pts with relapsed or refractory MZL treated with copanlisib from the Phase II CHRONOS-1 study (NCT01660451; Part B).
Methods: Pts enrolled in the open-label, single-arm CHRONOS-1 trial with histologically confirmed MZL who had relapsed after or were refractory to ≥2 prior systemic therapies, including rituximab and an alkylating agent, were included in the analysis. Copanlisib 60 mg was administered via a 1-hour i.v. infusion on an intermittent schedule of days 1, 8, and 15 of a 28-day cycle and continued until progression or unacceptable toxicity. The primary efficacy endpoint was ORR, defined as the proportion of pts who had CR or partial response as best response after ≥4 cycles, per independent assessment (Cheson BD et al. J Clin Oncol 2007). Secondary efficacy endpoints included duration of response (DoR), PFS, and overall survival (OS). Adverse events (AEs) were reported using MedDRA (v.20.1). The last pt was enrolled in Feb 2016, with an initial data cut-off of June 2016. The long-term follow-up reported here is based on a data cut-off of Feb 2018.
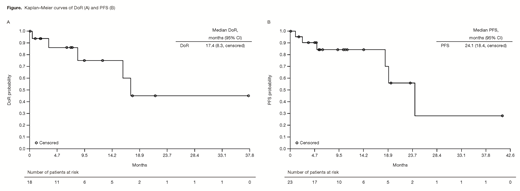
Results: Of the 23 MZL pts enrolled, 15 (65%) had nodal MZL, 4 (17%) had mucosa-associated lymphoid tissue (MALT) lymphoma, and 4 (17%) had splenic MZL. Pts had a median age of 69 years (range 39-81) and a median of 3 (range 2-9) prior systemic therapies; 48% were refractory to the last regimen and 44% were refractory to rituximab. As of Feb 2018, the median duration of copanlisib treatment was 23 weeks (range 1-191), and pts received a median of 5.8 cycles of treatment. Overall, 18 pts achieved an objective response (ORR 78%); CRs were observed in 3 (13%) pts, all with splenic MZL (3/4 pts). ORRs were 87% (13/15 pts) in nodal MZL, 75% (3/4 pts) in splenic MZL, and 50% (2/4 pts) in MALT MZL. Overall median DoR was 17.4 mo (range 0-37.6), with an estimated 45% in response after 2 years (Figure A) and a median DoR of 16.1 mo (range 0-37.6) in nodal MZL. Median PFS was 24.1 mo (range 0-41.1) (Figure B). Median OS was not reached (range 0.2-44.1) and estimated survival rate at 720 days was 83%. Of the 18 responding pts, median PFS was 24.2 mo (range 2.3-41.1); once remission was achieved, median DoR was 17.4 mo (range 0-37.6), with ~45% in remission beyond 2 years. The most common treatment-emergent AEs (TEAEs; all grade/grade 3+) were fatigue (52.2%/13.0%), hyperglycemia (47.8%/39.1%), and diarrhea (47.8%/13.0% all grade 3). Five pts had AEs leading to a dose reduction. The most common drug-related TEAEs were hyperglycemia (47.8%/39.1%), hypertension (43.5%/39.1% all grade 3), diarrhea (26.1%/13.0% all grade 3), and neutropenia (26.1%/8.7%). Hyperglycemia and hypertension were infusion related and were transient and manageable. Laboratory toxicities of interest were principally grade 1, including increased aspartate transaminase (31.8% all grade/22.7% grade 1) and increased alanine transaminase (27.3%/13.6%). No grade 5 TEAEs were reported. Tumor gene expression analysis from a subset of MZL pts demonstrated anti-tumor activity of copanlisib in MZL tumors characterized by PI3K pathway activation and/or B-cell receptor signaling.
Conclusions: Copanlisib demonstrated a promising ORR of 78% (CR 13%) and a manageable safety profile in pts with MZL who had received ≥2 prior systemic therapies. Responses were durable, with a median DoR of 17.4 mo and PFS of 24.1 mo, exceeding previous benchmarks. Phase III studies are underway to confirm the efficacy and safety of copanlisib in combination with other therapies in pts with indolent lymphoma, including MZL.
Dreyling:Acerta: Other: Scientific advisory board; Bayer: Other: Scientific advisory board, Speakers Bureau; Celgene: Other: Scientific advisory board, Research Funding, Speakers Bureau; Gilead: Other: Scientific advisory board, Speakers Bureau; Janssen: Other: Scientific advisory board, Research Funding, Speakers Bureau; Mundipharma: Other: Scientific advisory board, Research Funding; Novartis: Other: Scientific advisory board; Roche: Other: Scientific advisory board, Research Funding, Speakers Bureau; Sandoz: Other: Scientific advisory board. Panayiotidis:Bayer: Other: Support of clinical trial. Follows:Abbvie: Consultancy, Honoraria, Speakers Bureau; Janssen: Consultancy, Honoraria, Speakers Bureau; Roche: Consultancy, Honoraria, Speakers Bureau; AZ: Consultancy, Honoraria, Speakers Bureau. Özcan:Novartis: Research Funding; BMS: Other: Travel; Janssen: Research Funding, Travel; AbbVie: Research Funding; MSD: Research Funding; Celgene: Research Funding; Amgen: Honoraria; F. Hoffmann-La Roche Ltd: Other: Travel, Research Funding; Archigen: Research Funding; Takeda: Honoraria, Research Funding; Bayer: Research Funding; Jazz: Other: Travel; Sanofi: Other: Travel; Abdi Ibrahim: Other: Travel. Santoro:Arqule: Consultancy, Speakers Bureau; AstraZeneca: Speakers Bureau; Pfizer: Consultancy, Speakers Bureau; Takeda: Speakers Bureau; BMS: Speakers Bureau; Roche: Speakers Bureau; Abb-Vie: Speakers Bureau; Amgen: Speakers Bureau; Celgene: Speakers Bureau; Servier: Consultancy, Speakers Bureau; Gilead: Consultancy, Speakers Bureau; Lilly: Speakers Bureau; Sandoz: Speakers Bureau; Eisai: Consultancy, Speakers Bureau; Novartis: Speakers Bureau; Bayer: Consultancy, Speakers Bureau; MSD: Speakers Bureau; BMS: Consultancy. Stevens:Astellas: Consultancy. Hiemeyer:Bayer AG: Employment. Liu:Bayer Healthcare Pharmaceuticals, Inc.: Employment. Garcia-Vargas:Bayer Healthcare Pharmaceuticals, Inc.: Employment. Childs:Bayer Healthcare Pharmaceuticals, Inc.: Employment. Zinzani:Verastem: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Immune Design: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Portola: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Kyowa Kirin: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; TG Therapeutics: Honoraria, Speakers Bureau; MSD: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sanofi: Consultancy; Sandoz: Membership on an entity's Board of Directors or advisory committees; Servier: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen-Cilag: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Eusapharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celltrion: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.
Copanlisib received U.S. FDA Breakthrough Designation in May 2019 for the treatment of adult patients with relapsed marginal zone lymphoma who have received at least 2 prior therapies, though copanlisib is not currently approved for this indication.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal