Introduction: Classical Hodgkin lymphoma (cHL) typically affects younger patients but 15-35% are >60 years. The age used to define an elderly population has varied but age 60 is frequently used. A clinically relevant definition of older age could be based on the use of alternate treatments due to different efficacy and/or toxicity. Treatment outcomes may also be influenced by tumor biology and patient comorbidity that vary with age. We evaluated the effect of age on treatment outcomes in cHL.
Methods: All cHL patients treated at our centre between Jan 1999 and Dec 2015 were retrospectively analyzed. Clinical data were obtained from prospectively collected Lymphoma database and additional data was manually retrieved. Treatment for localized disease was combined modality (2-4 cycles of ABVD and potentially 6 cycles for bulk disease > 10 cm; radiation doses 20-35 Gy) with advanced disease typically receiving chemotherapy alone (ABVD 6-8 cycles). Older patients received individualized treatment. We used the Hematopoietic Cell Transplantation-specific Comorbidity Index (HCT-CI), (Sorror Blood 2005) as the elements can be abstracted retrospectively.
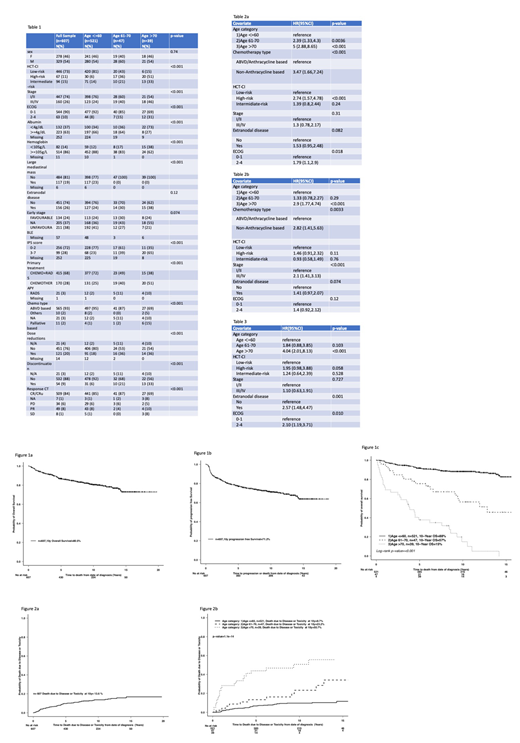
Results: 607 patients were identified; 14% were >60 years and 6% were age >70. Baseline characteristics are outlined in table 1. Patients >70 presented more frequently with high-risk HCT-CI and worse ECOG PS. Patients > 60 presented more frequently with advanced stage (61-70 age group: 40%; >70 years: 46%). 65% of the patients age >70 presented with an IPS of >3.
Chemotherapy alone approaches were used more commonly in older patients (age 61-70: 40%; age 70+: 51%) than in those <60 years (25%). Within the whole cohort 12 patients received non-anthracycline based treatment (<60: n=4; 61-70: n=1; and >70: n=7).For patients <60 and >70 this decision was made due to prior comorbidities that precluded the use of standard treatment, and for the patient in the 61-70 was because of acute toxicity with ABVD-based chemotherapy. Treatment was discontinued in 33% of the patients > 70 (77% due to toxicity), 21% in the 61-70 years group (30% toxicity) and 6% in patients <60 (29% toxicity). Patients > 70 had higher rates of grade 3-5 febrile neutropenia (28% versus 15% [age 61-70] and 7% [age <60]). Bleomycin toxicity was more common in older patients (age >70: grade 3-4 events 12% of the patients with discontinuation in 66%; age 61-70: 13% with discontinuation rate of 20%) compared to a 1% rate of grade 3-5 events in age <60. There was a grade 5 episode of febrile neutropenia in >70 group and 1 death related to bleomycin in age <60.
With a median follow up of 8.6 years, the 10-year OS and PFS were 80.5% and 71.2%, respectively. By age-group, the 10-year OS was 88% (<60 years), 57% (61-70 years) and 15% (age >70 years); (p<0.001) (Figure 1a-b). In multivariable analysis for OS, age 61-70 (HR 2.44, p=0.002) and age >70 (HR 5.72, p=0.001), non-anthracycline based chemotherapy (HR 3.69, p<0.001), high-risk HCT-CI (HR 3.03, p=0.001) and ECOG 2-4 (HR 1.8, p=0.017), were significant. Age >70, type of chemotherapy, high-risk HCT-CI, and advanced stage were significant for PFS in the multivariable analysis (Table 2a-b).
Death due to disease or toxicity at 10 years was 13.6% (age <60: 9.7%, 61-70 years: 23.2%; and for age > 70: 50.7%; [p=<0.001]) (Figure 2a-b). Multivariable analysis for cause-specific survival identified age >70 years (HR 4.04, p=<0.001), extranodal disease (HR 2.57, p=0.001) and ECOG 2-4 (HR 2.10, p=0.010) as significant predictors(Table 3).
Conclusions: Age >70 years is a clinically relevant age cutoff as it has additional prognostic significance and greater rates of treatment discontinuation and toxicity compared to age 60. The HCT-CI is a useful predictor of outcome in cHL and should be validated prospectively. In multivariable analysis, age, type of treatment, comorbidity and ECOG performance status are independent predictors of OS. Further studies are ongoing to validate these findings and assess biologic differences in older versus younger cHL patients.
Tsang:Nordic Nanovector: Research Funding. Kridel:Gilead Sciences: Research Funding. Kukreti:Celgene: Honoraria; Amgen: Honoraria; Takeda: Honoraria. Prica:Celgene: Honoraria; Janssen: Honoraria. Kuruvilla:Janssen: Research Funding; Roche: Honoraria; Novartis: Honoraria; Merck: Honoraria; Gilead: Honoraria; Seattle Genetics: Consultancy; Roche: Consultancy; Merck: Consultancy; Karyopharm: Consultancy; Celgene: Honoraria; BMS: Honoraria; BMS: Consultancy; Abbvie: Consultancy; Gilead: Consultancy; Astra Zeneca: Honoraria; Janssen: Honoraria; Roche: Research Funding; Amgen: Honoraria; Seattle Genetics: Honoraria; Karyopharm: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal