Introduction: ET is a chronic myeloproliferative neoplasm (MPN) characterized by thrombocytosis and an increased risk for thrombotic and hemorrhagic events. ET can be associated with substantial symptom burden, impaired quality of life (QoL), and reduced survival. PRO data pertaining to the impact of ET on QoL and symptom burden in these pts are limited. The ongoing Myelofibrosis and Essential Thrombocythemia Observational STudy (MOST) was designed to collect data about the demographics, disease burden, PROs, and management of pts with ET or myelofibrosis (MF) in clinical practices throughout the United States. This analysis describes PROs from pts with ET enrolled in MOST.
Methods: MOST is a longitudinal, multicenter, noninterventional, prospective, observational study (NCT02953704). Eligible adults with ET were ≥60 years of age, had a history of thrombotic events, or were receiving ET-directed therapy. PROs were collected in conjunction with usual-care visits approximately every 6 months over a planned observation period of 36 months. Patient-reported symptom burden was assessed with the disease-specific MPN Symptom Assessment Form Total Symptom Score (MPN-SAF TSS), composed of 10 items (fatigue, early satiety, abdominal discomfort, inactivity, concentration problems, night sweats, itching, bone pain, fever [>100oF], weight loss). The MPN-SAF numbness/tingling item was also included in the questionnaire but was not included in the TSS calculation. Symptom severity was graded from 0 (absent) to 10 (worst imaginable), with a possible TSS ranging from 0 to 100. Health-related QoL was evaluated with the European Organization for Research and Treatment of Cancer Core Quality of Life Questionnaire (EORTC QLQ-C30 v3.0), composed of 5 functional scales, 3 symptom scales, 6 additional single-symptom items, and a global health/QoL scale. For functional and global health/QoL scales, higher scores indicate higher functioning and better global health/QoL, respectively. For symptom scales/items, higher scores indicate greater symptom burden. High-risk pts and low-risk pts receiving ET-directed therapy (excluding aspirin only) with baseline PRO data were included in this analysis. Data were summarized with descriptive statistics.
Results: The MOST study enrolled 1234 pts with ET between Nov 29, 2016 and March 29, 2019 at 124 sites. Of these pts, 794 qualified for this analysis (data cut-off date, June 17, 2019); median age was 70 (range, 19-93) years, 80% were ≥60 years of age, 68% were women, 90% were white, 42% were working full or part-time, and 4% had a documented family history of MF, ET, or polycythemia vera. The majority of pts (87%) had high-risk ET.
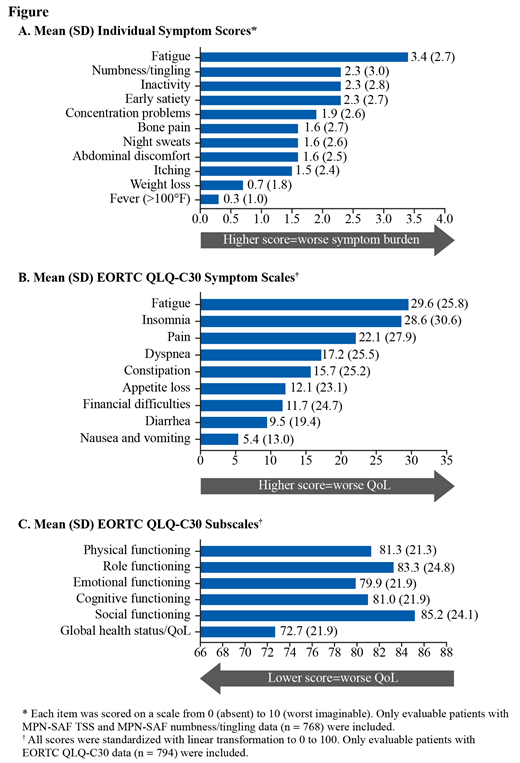
At enrollment, 768 pts completed the MPN-SAF. Mean (SD) TSS was 17.1 (15.6); 33% of pts had TSS ≥20. Women had higher mean (SD) TSS than men (18.5 [15.8] vs 14.2 [14.9]) and had higher mean individual symptom scores, except for weight loss and fever. The highest mean (SD) individual symptom scores were fatigue (3.4 [2.7]), numbness/tingling (2.3 [3.0]), inactivity (2.3 [2.8]), and early satiety (2.3 [2.7]) (Fig A). The most frequently reported severe symptoms (ie, score ≥7) were fatigue (17% [127/746]), numbness/tingling (14% [107/767]), and inactivity (11% [86/762]).
At enrollment, 794 pts completed the EORTC QLQ-C30. The highest mean (SD) symptom scale scores (score ≥15) were fatigue (29.6 [25.8]), insomnia (28.6 [30.6]), pain (22.1 [27.9]), dyspnea (17.2 [25.5]), and constipation (15.7 [25.2]) (Fig B). The mean (SD) global health status/QoL score was 72.7 (21.9); functional scores ranged from 79.9 (21.9) for emotional functioning to 85.2 (24.1) for social functioning (Fig C). The average functional scale scores and symptom scale scores indicate higher functioning and less symptom burden, respectively, in men vs women.
Conclusion: Pts with ET experienced a high symptom burden; fatigue was the most common and highest in severity. Symptom burden and quality of life scores in the current study were similar to prior reports (Emanuel J Clin Oncol 2012; Scherber Blood 2011). Women reported higher symptom burden than men in both the MPN-SAF and EORTC QLQ-30. Of note, numbness/tingling, which is not included in the MPN-SAF TSS calculation, was one of the most frequently reported severe symptoms for pts with ET in MOST. Future analyses from this trial will continue to increase understanding of the symptom burden and its impact on QoL in pts with ET.
Ritchie:Celgene, Incyte, Novartis, Pfizer: Consultancy; Genentech: Other: Advisory board; Tolero: Other: Advisory board; Pfizer: Other: Advisory board, travel support; agios: Other: Advisory board; Celgene: Other: Advisory board; Jazz Pharmaceuticals: Research Funding; Celgene, Novartis: Other: travel support; AStella, Bristol-Myers Squibb, Novartis, NS Pharma, Pfizer: Research Funding; Ariad, Celgene, Incyte, Novartis: Speakers Bureau. Al-Janadi:Incyte: Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Celgene: Honoraria, Other: Travel, Accommodations, Expenses, Research Funding, Speakers Bureau; Genentech/Abbvie: Honoraria, Other: Travel, Accommodations, Expenses, Speakers Bureau; Genentech/Roche: Honoraria, Other: Travel, Accommodations, Expenses, Speakers Bureau; Gilead Sciences: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses; Sandoz-Novartis: Consultancy, Honoraria; Alexion Pharmaceuticals: Consultancy, Honoraria, Other: Travel, Accommodation, Expenses, Research Funding, Speakers Bureau; Takeda: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding, Speakers Bureau; MEI Pharma: Research Funding; Seattle Genetics: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses. Colucci:Incyte: Employment, Equity Ownership. Kalafut:Incyte: Employment, Equity Ownership. Paranagama:Incyte: Employment, Equity Ownership. Mesa:Genotech: Research Funding; Promedior: Research Funding; Sierra Onc: Consultancy; Celgene: Research Funding; AbbVie: Research Funding; Novartis: Consultancy; La Jolla Pharma: Consultancy; CTI Biopharma: Research Funding; Samus: Research Funding; Incyte: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal