Introduction: Bone marrow stromal cells (BMSCs), a major component of CLL microenvironment influences CLL B-cell survival. We have utilized an in vitro BMSC model system and found unique alterations in CLL B-cells with short term BMSC co-culture that point to previously unidentified biologic changes in the CLL B-cells that may influence CLL B-cell signaling and their drug resistance.
Methods: Purified primary CLL B-cells (n=41) from previously untreated CLL patients were cultured alone or co-cultured with primary BMSCs from either normal individuals (n=24) or CLL patients (n=18) at a 50:1 ratio in AIMV medium. After 48 hours, separated CLL B-cells or BMSCs were examined by immunoprecipitation/Western blot analyses to assess the presence of intracellular signal proteins and real time PCR to determine the RNA level expression. In separate experiments to assess CLL B-cell response in co-culture with BMSC, purified CLL B-cells were treated with the following agents at sub lethal doses; fludarabine, chlorambucil, ibrutinib and venetoclax as single agents.
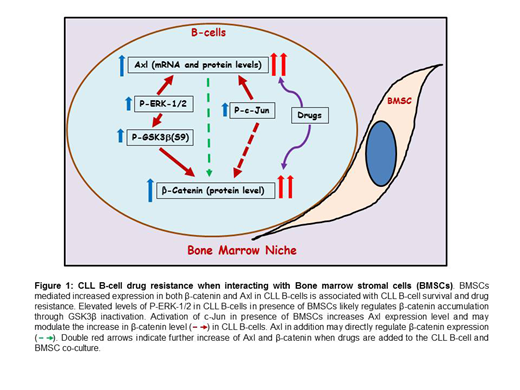
Results: Consistent and significant increase in expression of Axl receptor tyrosine kinase for both mRNA and protein levels was observed in CLL B-cells co-cultured with BMSCs compared to CLL B-cells cultured alone. We also detected significantly increased expression of β-catenin at the protein level in CLL B-cells co-cultured with BMSCs. In contrast there was no significant change in β-catenin or Axl protein expression in the co-cultured BMSCs. Co-culturing of CLL B-cells with BMSCs using transwells confirmed that the upregulation of both Axl and β-catenin is dependent on the direct contact of CLL B-cells with BMSCs. Experiments showed that the increased Axl level in co-cultured CLL B-cells was independent of Axl kinase activity. Furthermore, the CLL B-cells from co-culture also had clear upregulation of downstream P-ERK-1/2 but no change in P-AKT(Ser473).
Next we reasoned that if increases in Axl and β-catenin are related to drug resistance then CLL B-cells should have increases in those proteins when cultured with drugs and in presence of BMSCs. Treatment with chemotherapeutic or targeted therapy drugs, (i.e. fludarabine, chlorambucil, ibrutinib, venetoclax) with sublethal doses was found to lead to increase in expression levels of both β-catenin and Axl in co-cultured CLL B-cells. However these increases were significantly over that seen with simple co-culture of CLL B-cells with BMSCs. Since CLL B-cells were less sensitive to these latter chemotherapy drugs in presence of BMSCs, this suggests to us a role for both Axl and β-catenin in stromal mediated CLL B-cell drug resistance to these agents.
Moreover, high nuclear β-catenin and P-ERK-1/2 levels were also detected in co-cultured CLL B-cells. It is also known that ERK associates with and inactivates GSK-3β resulting in the up-regulation of β-catenin. We found upregulation in P-GSK-3β (Ser9) an inactive molecule which can result in increasing accumulation of β-catenin. Inhibition of P-ERK-1/2 using the ERK inhibitor PD98059 in co-cultured CLL B-cells inhibited β-catenin as well as Axl expression levels. Furthermore, it has been shown that Axl expression can be regulated by c-Jun activity. In that regard we have observed upregulation of P-c-Jun(Ser73) which can enhance Axl level in co-cultured CLL B-cells and inhibition of c-Jun activity using SP600125 (c-jun upstream JNK inhibitor), as well inhibited the Axl expression.
Finally, we studied 5 CLL patients before and while being treated with ibrutinib/chemo-immuno therapy for the expression of CLL B-cell Axl, β-catenin, P-ERK-1/2, P-c-Jun(Ser73) levels. We found that 2 patients had increase in Axl expression, 4 patients had increased β-catenin and P-ERK-1/2 levels and 3 patients showed increase in P-c-Jun(S73) level after the therapy.
Conclusion: Here we show that marrow stromal cell contact with CLL B-cells consistently mediate increased expression in both β-catenin and Axl in CLL B-cells (Figure1). The mechanism for this may, in part, via activated ERK and c-Jun levels (Figure1). We believe that these changes in both molecules are associated with leukemic B-cell survival and drug resistance (Figure1). These studies suggest that a further understanding of the roles of Axl and β-catenin in the CLL B-cells mediated by contact with BMSC will help to develop potential strategies for the management of CLL disease resistant to various drugs.
Warner:Tolero Pharmaceuticals: Employment. Bearss:Tolero Pharmaceuticals: Employment. Kay:Agios: Other: DSMB; Infinity Pharmaceuticals: Other: DSMB; Celgene: Other: Data Safety Monitoring Board; MorphoSys: Other: Data Safety Monitoring Board.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal