Background: Patients with multiple myeloma (MM) who have relapsed after conventional treatment have limited therapeutic options for long-term disease control. Melflufen is a lipophilic peptide-conjugated alkylator that rapidly delivers a highly cytotoxic payload into myeloma cells through peptidase activity. In the first report of efficacy and safety for the phase 1/2 study O-12-M1 (median follow-up, 28 months), melflufen and dexamethasone demonstrated an overall response rate of 31%, a median PFS of 5.7 months, and a median OS of 20.7 months, with acceptable safety for patients with RRMM (Richardson PG, et al. Blood. 2017; Abstract 3150). Here, updated OS and PFS results from the O-12-M1 study are reported, with 18 months of additional follow-up of the patients who were still participating in long-term follow-up at the time of the final database lock in November 2017.
Methods: Eligible patients had RRMM, measurable disease, and ≥2 prior lines of therapy, including bortezomib and lenalidomide. Patients must have had progressive disease (PD) on or within 60 days of completion of last therapy. Patients received melflufen 40 mg intravenously on day 1 of each 28-day cycle and oral dexamethasone 40 mg weekly for up to 8 cycles or longer at the discretion of the investigator and sponsor. Treatment continued until PD or unacceptable toxicity. Patients were followed up for 2 years after PD or start of subsequent therapy. PFS and OS were secondary end points in this study. Time to next treatment (TTNT), an exploratory end point defined as the time from start of melflufen and dexamethasone to first subsequent therapy or death, was retrospectively reviewed.
Results: As of 15 May 2019, 45 patients were treated. Median age was 66 years (range, 47-78); 60% of patients had International Staging System stage II/III at study entry, and 44% had high-risk cytogenetics [del(17p), t(14;16), t(4;14), t(14;20), or gain(1q)]. The median time since initial diagnosis was 5.0 years (range, 1-21). Patients received a median of 4 prior lines of therapy (range, 2-14). All patients were exposed to IMiDs, 98% to proteasome inhibitors (PIs), 93% to alkylators (any dose of melphalan, cyclophosphamide, or bendamustine), and 80% to melphalan; 87% were refractory to last line of therapy, and 91%, 67%, and 7% were single (IMiD or PI), double (IMiD and PI), and triple (IMiD, PI, and daratumumab) refractory, respectively.
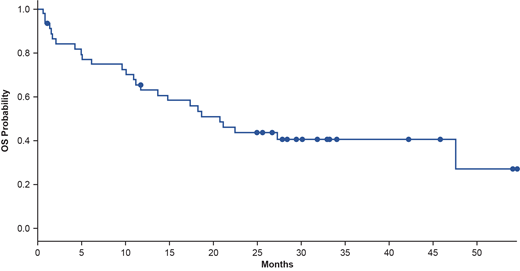
After a median follow-up of 30.1 months, median PFS was 5.7 months (95% CI, 3.7-9.3; 98% events). Median OS was 20.7 months (95% CI, 13.6-not reached; 58% events; Figure). Updated PFS, OS, and TTNT data will be presented. No new adverse events (AEs) were reported.
Conclusion: Melflufen and dexamethasone resulted in sustained long-term benefits (median OS, 20.7 months) and no new AEs with 1.5 years of additional follow-up of patients with late-stage, heavily pretreated RRMM who have relapsed on conventional therapy including bortezomib and lenalidomide. Further trials are ongoing to evaluate efficacy and safety of melflufen, including the phase 3 study OCEAN (OP-103; NCT03151811) of melflufen plus dexamethasone versus pomalidomide plus dexamethasone in patients with RRMM refractory to lenalidomide.
Bringhen:Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Honoraria; Celgene Corporation: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees. Voorhees:Novartis: Consultancy; Oncopeptides: Consultancy; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; TeneoBio: Consultancy, Research Funding; Amgen: Research Funding; GSK: Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Membership on an entity's Board of Directors or advisory committees. Plesner:AbbVie: Consultancy; Genmab: Consultancy; Takeda: Consultancy; Oncopeptides: Consultancy; Celgene: Consultancy; Janssen: Consultancy, Research Funding. Mellqvist:Amgen, Janssen, Oncopeptides, Sanofi, Sandoz, Takeda: Honoraria. Reeves:Celgene: Honoraria, Speakers Bureau; Incyte: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Seattle Genetics: Consultancy, Honoraria. Sonneveld:Amgen: Honoraria, Research Funding; SkylineDx: Research Funding; Takeda: Honoraria, Research Funding; Karyopharm: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; BMS: Honoraria. Byrne:Oncopeptides: Consultancy; Takeda: Consultancy. Nordström:Oncopeptides: Employment, Equity Ownership. Harmenberg:Oncopeptides: Consultancy, Equity Ownership. Obermüller:Oncopeptides: Employment. Richardson:Sanofi: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Oncopeptides: Membership on an entity's Board of Directors or advisory committees, Research Funding; Karyopharm: Membership on an entity's Board of Directors or advisory committees.
This is a Phase 1/2 investigational study of melflufen in RRMM
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal