Introduction: Multiple myeloma (MM) is a heterogeneous malignant plasma cell (PC) disorder and the survival ranges from several months to > 10-years. Revised International Staging System (R-ISS) has been reported to define subgroups of patients with MM with different prognosis, however, most of the patients were classified as R-ISS stage II, thus, it includes a heterogenous population with various risk factors. 18F-fluoro-deoxyglucose (18F-FDG) positron emission tomography/computed tomography (PET/CT) allows the direct assessment of metabolic tumor burden in various malignancies. Therefore, metabolic tumor volume (MTV) and total lesion glycolysis (TLG), which are volumetric parameters applicable to 18F-FDG PET/CT, are emerging tools for MM prognostication. This study aimed to determine the value of MTV and TLG using 18F-FDG PET/CT in the prognostication and to determine the high-risk patients in R-ISS stage II in combination with R-ISS and MTV and TLG.
Methods: A total of 191 consecutive patients with newly diagnosed MM (NDMM) who underwent baseline whole-body 18F-FDG PET/CT and R-ISS between January 2009 and June 2019 at Kameda Medical Center, Kamogawa-shi, Japan, were retrospectively analyzed. Del(17p), translocation t(4; 14), and t(14;16) detected by interphase fluorescent in situ hybridization were considered high-risk chromosomal abnormalities. All patients underwent combination chemotherapy using novel agents. 18F-FDG PET/CT was performed using dedicated PET/CT scanners (Discovery ST Elite Performance; GE Healthcare, Milwaukee, USA). The standard uptake value (SUV) was normalized according to the injected dose and lean body mass. The baseline SUVmax of all lesions was recorded, and the highest value was considered as the SUVmax of the patient. MTV was defined as the myeloma lesions volume visualized on PET/CT scans with SUV greater than or equal to the fixed absolute threshold of SUV = 2.5. TLG was calculated as the sum of the product of average SUV (SUVmean) and MTV of all lesions. Computer‐aided analysis of PET/CT images for MTV and TLG calculations was performed using an open-source software application of Metavol (Hokkaido University, Sapporo, Japan). Nine patients were excluded because MTV data could not be retrieved. Ultimately, 182 patients were included in our analysis. Written informed consent was obtained from all patients.
Results: Whole-body MTV and TLG ranged from 0 to 2440.7 mL (median; 34.2 mL) and from 0 to 12582.4 g (median; 97.0 g). The best cut-off values of MTV and TLG that discriminate the survival using a receiver-operating-characteristic curve analysis were 56.4 mL and 166.4 g, respectively. Both MTV and TLG can be used to stratify patients according to cut-off values using Kaplan-Meier survival curves and log-rank test. At a median follow-up of 32 months, the overall survival (OS) and progression-free survival (PFS) of patients with a lower cut-off value of MTV (≤56.4 mL) had better survival compared to those with a higher cut-off value (>56.4 mL) [not reached (NR) vs 52.9 months for OS, p=0.003, and 37.3 vs 23.8 months for PFS, p=0.019, respectively]. Similarly, the OS and PFS of patients with a baseline lower cut-off value of TLG (≤166.4 g) showed better survivals compared to those with a higher cut-off value (>166.4 g) (NR vs 54.3 months for OS, p=0.0047, and 37.3 vs 28.8 months for PFS, p=0.012, respectively).
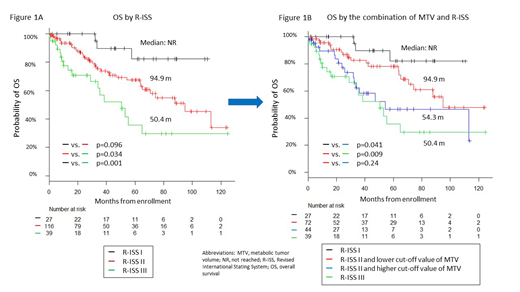
Although R-ISS can stratify the OS of patients into 3 groups (Figure 1A), we explored the clinical variables and MTV and TLG to further identify the high-risk patients among the R-ISS stage II. We divided the following 4 groups: R-ISS stage I; R-ISS stage II with a lower cut-off value of MTV or TLG; R-ISS stage II with a higher cut-off value of MTV or TLG; and R-ISS stage III. The median OS was NR in R-ISS stage I, 94.9 months in R-ISS stage II with a lower cut-off value of MTV, 54.3 in R-ISS stage II with a higher cut-off value of MTV, and 50.4 in R-ISS stage III (Figure 1B); the median PFS were NR, 32.0, 30.2, and 29.3 months, respectively. Other clinical factors including age, hemoglobin, creatinine, and stem cell transplantation were not associated with the prognosis of these patients.
Conclusion: Our findings demonstrated that MTV and TLG calculated from pretreatment PET/CT were useful for risk stratification in patients with NDMM. By identifying patients with stage II and a poor prognosis, it may be possible to select patients who are candidates for aggressive treatment.
Matsue:Janssen Pharmaceutical K.K.: Honoraria; Novartis Pharma K.K: Honoraria; Ono Pharmaceutical: Honoraria; Takeda Pharmaceutical Company Limited: Honoraria; Celgene: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal