Background: CLR 131 [18-(p-[131I]-iodophenyl)octadecyl phosphocholine] is an investigational, radioiodinated cancer therapy that exploits the selective uptake and retention of phospholipid ethers (PLEs) and PLE analogs by malignant cells [Mollinedo 2010, Weichert 2014]. To produce CLR 131, the core PLE analog is radioiodinated with the radioisotope iodine-131 (I-131).
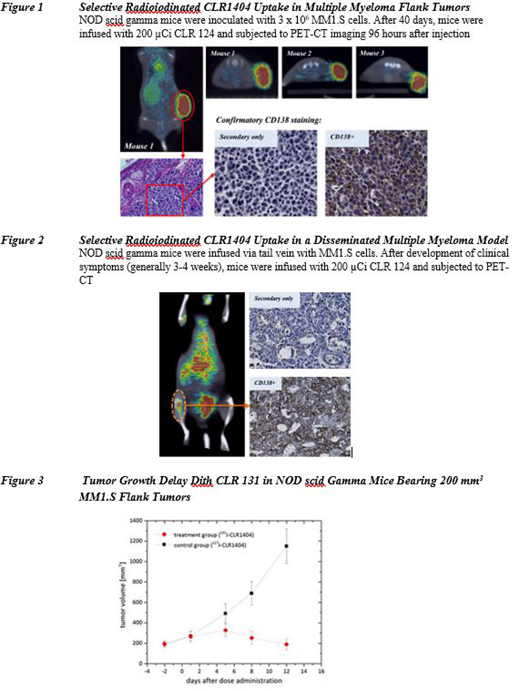
CLR 131 provides targeted delivery of radiation to malignant tumor cells, thus minimizing radiation exposure to normal tissues. The mechanistic basis for the cancer-cell selective uptake involves interaction with lipid raft regions of the plasma membrane. Imaging studies with CLR 124 [18-(p-[124I]-iodophenyl)octadecyl phosphocholine], which is chemically and structurally identical to CLR 131, have demonstrated cancer-cell selective uptake and retention in all but 3 of over 60 tumor cell models assessed to date [Weichert 2014]. Using a MM1.S myeloma xenograft murine model, highly selective uptake of CLR 124 was documented using I-124 PET imaging (Figure 1). Similarly, using CLR 124 in a disseminated MM1.S myeloma model, PET-CT documented selective uptake of CLR1404 in anatomic regions of dense myelomatous involvement (Figure 2). CLR 131 activity was assessed in NOD scid gamma mice bearing 200 mm3 MM1.S flank tumors who received a single tail vein injection of CLR 131 (100 µCi) or a mass equivalent dose of CLR1404. As anticipated, a single dose of CLR 131 produced tumor growth delay (Figure 3).
Study Design and Methods:
This Phase 1 trial (NCT02278315) is assessing CLR 131 in patients with relapsed or refractory multiple myeloma (RRMM). The primary objective is to determine the safety and tolerability of CLR 131 as a single or multiple dose, with and without concurrent weekly dexamethasone, in patients with RRMM who have previously been treated with, or are intolerant of, an immunomodulator and a proteasome inhibitor. Secondary objectives include identifying the recommended Phase 2 dose and schedule, and determining therapeutic activity.
Eligibility criteria include progressive, RRMM, and at least one previous exposure to proteasome inhibitor and immunomodulatory drugs with no limit to the number of prior lines of therapy. Patients are to have at least 5% plasma cell involvement and measurable disease (by m-protein or FLC, although non-secretors are considered on a case-by-case basis). Patients are excluded if they have had prior radioisotope therapy, prior total body or hemi-body irradiation, or prior external beam radiation therapy resulting in greater than 20% of total bone marrow receiving greater than 20 Gy.
Patients receive CLR 131 as a fractionated infusion of CLR 131 at increasing doses administered on day 1 and day 7 (± 1 day) as a 30-minute intravenous infusion with 40 mg concurrent weekly dexamethasone. All patients take thyroid protection medication starting the day prior to CLR 131 infusion and continuing for 14 days after the last CLR 131 infusion. Following the CLR 131 infusions, patients are followed for a total of 12 weeks to assess safety and efficacy.
At least 3 to 4 patients are enrolled in each dose level. Patients who discontinue prior to the day 64 assessment can be replaced. A Data Monitoring Committee evaluates each dose level for safety and tolerability and provides recommendations for dose escalation, de-escalation, or expansion.
Current Status As of 31-Jul-2019, 28 subjects have been enrolled; 18 patients received a single infusion of CLR 131 and 10 patients have received fractionated doses of CLR 131. Enrollment in the 40 mCi/m2 fractionated dose level is ongoing.
Longcor:Cellectar Biosciences: Employment, Equity Ownership. Oliver:Cellectar Biosciences: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal