Background: Few registry studies have investigated the use of cord blood transplantation (CBT) for treatment of patients with myelodysplastic syndrome (MDS) and myeloproliferative disorders (MPN). To date, the outcomes reported have been not encouraging with excess of graft failures [1] and poor disease-free survival (DFS) [2]. The major obstacle to the success of CBT in this high-risk population has been related to the high rate of non-relapse mortality (NRM) due to the delayed time to engraftment. In this single-center study we retrospectively investigated clinical outcomes in patients with MDS and MPN undergoing a CBT.
Methods: We reviewed clinical outcomes in 65 consecutive patients with either MDS (n=42) or MPN (n=23) who received a CBT at our institution between 2006 and 2018. The conditioning regimens used consisted of Cyclophosphamide (CY), Fludarabine (FLU), and total body irradiation (TBI) 13.2 Gy (n=16); Treosulfan, FLU, and TBI 2 Gy (n=26); Busulfan, CY, and FLU (n=6); or CY, FLU, and TBI (2 to 4 Gy) (n=17). All patients received Cyclosporine and Mycophenolate Mofetil for graft-versus-host disease (GVHD) prophylaxis. Cord blood units were HLA-typed at the antigen level for HLA-A and B, and high resolution for HLA-DRB1. Disease risk was classified as low to intermediate (n=37) and high to very high (n=28). Cytogenetics risk at diagnosis was favorable, intermediate and unfavorable in 11 (17%), 33 (51%) and 21 (32%) patients, respectively. Among patients with MDS, the R-IPSS score was low in 6 (14%), intermediate in 18 (43%), high in 8 (19%), and very-high in 10 (24%). Engraftment, relapse, NRM, and acute GVHD were calculated using cumulative incidence estimates adjusting for the appropriate competing risks. DFS and overall survival (OS) were assessed using the Kaplan-Meier method.
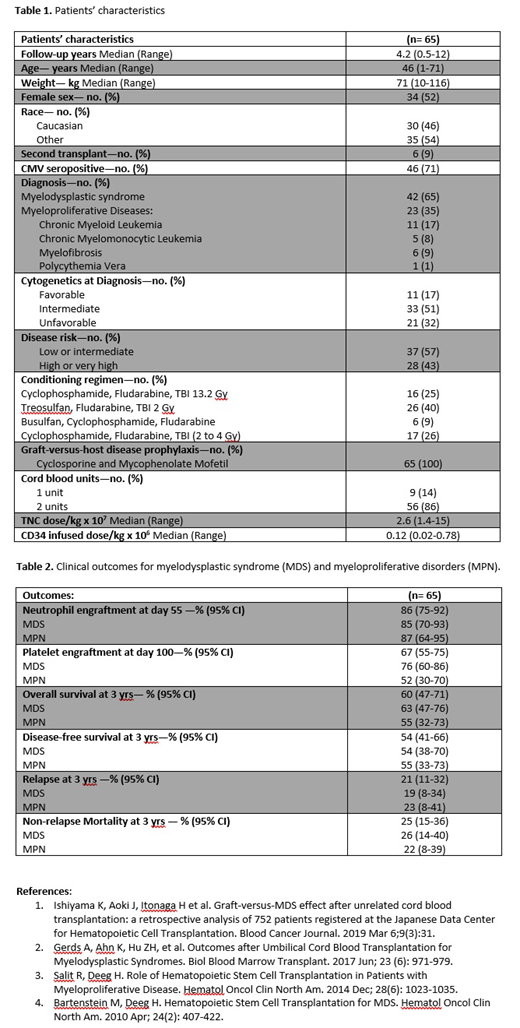
Results: Patient characteristics are shown in Table 1. Median follow-up was 4.2 years (range, 0.5-12). The median age and weight were 46 years (range, 1-71) and 71 kg (range, 10-116). Six (9%) patients had failed a prior HCT. 56 (86%) patients received 2 cord blood units to achieve the required cryopreserved cell dose. The median cell doses infused were 2.6 x 107 (range, 1.4-15) TNC/kg and 0.12 x 106 (range, 0.02-0.78) CD34+ cells/kg. Neutrophils (>500 for 3 days) and platelet (>20x109/L for 7 days transfusion free) engraftment occurred at a median time of 20 days (range, 6-89) and 35 days (range, 13-85), respectively. There were only 4 (6%) cases of primary graft failure, 2 of which were patients with MDS and 2 patients with MPN, and no cases of secondary graft failures. 76% of MDS patients and 52% of MPN patients achieved platelet engraftment by day 100. OS at 3 years was 60% (95% Confidence Interval, CI: 47-71) and specifically was 63% (95% CI: 47-76) for MDS patients and 55% (95% CI: 32-73) for MPN patients. Disease free survival at 3 years was 54% (95% CI: 41-66) with a cumulative incidence of relapse of 21% (95% CI: 11-32). The cumulative incidence of NRM at day 100 and at 3 years were 9% (95% CI: 4-17) and 25% (95% CI: 15-36), respectively. No differences in DFS, relapse incidence, and NRM were seen between MDS and MPN patients (Table 2). The cumulative incidence of acute GVHD grade II-IV and III-IV were 74% (95% CI: 61-82) and 21% (95% CI: 12-32), respectively. Seventeen (26%) patients developed late acute GVHD at a median time of 186 days (range, 101-379), and 10 (15%) had overlap syndrome. Twenty-three (35%) patients were diagnosed with classical chronic GVHD: 18 (27%) with mild, 3 (5%) with moderate, and only two (3%) with severe grade.
Conclusions: The results presented herein demonstrate that in patients with MDS and MPN undergoing CBT sustained engraftment was achievable without excessive graft failures. The rates of OS and DFS at 3 years were extremely encouraging and similar to what was observed after matched related and unrelated donors' transplants. [3,4]. Overall, our data suggest that CBT should be considered as a valid option for this high-risk population in case of lack of conventional donors.
Delaney:Biolife Solutions: Membership on an entity's Board of Directors or advisory committees; Nohla Therapeutics: Employment, Equity Ownership. Milano:ExCellThera: Research Funding; Amgen: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal