Background: Next-generation sequencing (NGS) technologies have led to the discovery of recurrently-mutated genes in acute myeloid leukemia (AML) and myelodysplastic syndromes (MDS). These mutations have proven useful in diagnosis, prognostication, and prediction of treatment response when used in conjunction with cytogenetics and other clinical characteristics known to influence patient outcome. NGS platforms that test for the presence of genetic mutations associated with myeloid malignancies are now widely available in clinical practice and are often utilized during initial diagnostic evaluation. However, there is considerable variability among hematologists in the use of such data, and it is still unclear whether physicians are using molecular data to properly prognosticate or make treatment decisions for their patients. Thus, the purpose of this study is to assess real-world practices of physicians caring for patients with AML or MDS at a large National Comprehensive Cancer Network (NCCN) Member Institution and to determine the degree to which molecular data is being utilized to prognosticate or alter clinical management.
Methods: Patients presenting to the University of Wisconsin-Madison Hospital and Clinics with newly-diagnosed AML or MDS from 1/1/2014 through 12/31/2018 were included in the study. Data was collected retrospectively, including patient demographics, clinical characteristics, cytogenetic and mutational data, and treatment. The medical record was scrutinized for documentation regarding impact of molecular data on physician prognostication and decision-making.
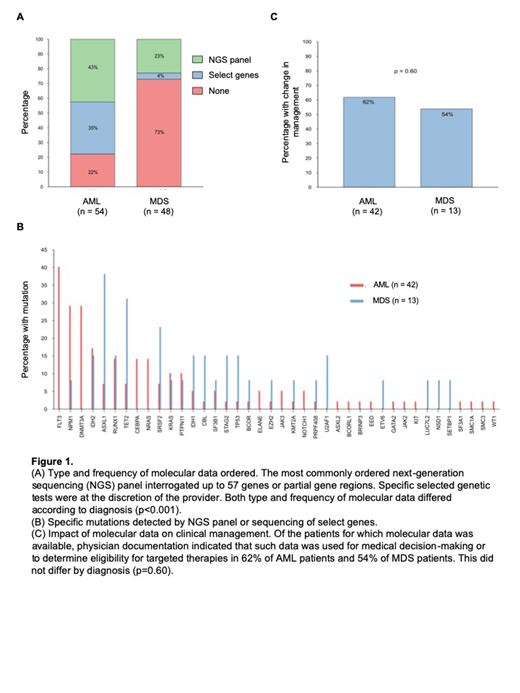
Results and Discussion: 102 patients were included in the analysis, including 54 patients with AML and 48 patients with MDS. For AML patients, 36% were classified as adverse risk, 34% as intermediate risk, and 30% as favorable risk based on ELN criteria. For MDS patients, 50% were classified as very high or high risk, 27% as intermediate risk, and 23% as low or very low risk by IPSS-R criteria. The proportion of AML and MDS patients having normal cytogenetics were comparable (37% versus 29% respectively, p=0.40). The type and frequency of molecular data utilized differed significantly according to diagnosis (p<0.001). Molecular data was obtained for 78% of patients with AML compared to 27% of patients with MDS. An expanded NGS panel was ordered for 43% of AML patients and 23% of MDS patients, whereas specific selected genetic tests were ordered for 35% of AML patients and 4% of MDS patients. The most commonly utilized NGS panel interrogated up to 57 genes or partial gene regions. There was no association between disease risk at presentation and odds of obtaining molecular data (p=0.16). 40 genes had identifiable mutations in the entire cohort with FLT3, NPM1, DNMT3A, IDH2, and ASXL1 mutations being the most common. Of those for which molecular data was obtained, physician documentation indicated such data was used for medical decision-making or rationale of treatment choice for 60% of patients, including 62% of AML patients and 54% of MDS patients. Based on molecular data, disease was perceived as lower risk in 6% of patients, higher risk in 42% of patients, and equivocal risk in 6% of patients; 42% of patients were found to be eligible for targeted therapies. Bone marrow transplantation (BMT) was performed in 41% of patients with AML and 31% of patients with MDS. The odds of undergoing BMT when molecular data was obtained was 4.2 times the odds of BMT in the absence of molecular data (95% CI 1.5-12, p=0.007), and this association was common to both diagnoses (p=0.29).
Conclusion: At our institution, molecular genetic sequencing was obtained in the majority of patients presenting with AML but less frequently in patients with MDS. This likely reflects the less-defined role of genetics in the classification of MDS and limited availability of targeted therapies. Disease risk at presentation did not influence whether a provider ordered molecular genetic testing. Molecular data was utilized to elucidate patient prognosis or determine candidacy for targeted therapies in nearly two thirds of patients who had molecular sequencing performed, and its utility did not differ by diagnosis. Novel to this study, we showed that the presence of molecular data was associated with an increased odds of pursuing BMT. Our study demonstrates the current extent to which molecular data impacts clinical management of patients with AML and MDS.
Lasarev:Ultragenyx: Consultancy. Mattison:Pfizer: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal