Introduction: Whether adults aged ≥60 years with AML benefit from multiagent (or intensive) chemotherapy is a matter of controversy. Prior studies have demonstrated conflicting results. We performed analysis of a large National Cancer Data Base (NCDB) to determine the value of multiagent vs. single agent chemotherapy.
Methods: NCDB captures approximately 70% of all cancer diagnoses in US, and undergoes rigorous quality monitoring. We utilized NCDB to identify patients aged 60-79 years, who were newly diagnosed with AML (other than APL) between the years 2004-2014. Logistic regression model was used to determine factors associated with the use of multiagent chemotherapy. Kaplan-Meier curves were generated and compared using the log rank test. Logistic regression model and Cox Proportional Hazard model were used for one-month mortality and OS analyses, respectively. In a separate analysis, patients who received single agent (n=6743) vs. multiagent chemotherapy (n=6743) were matched based on the variables age, Charlson comorbidity score, and AML subtypes (good-risk AML, therapy-related AML/AML with myelodysplasia related changes, and other intermediate/high-risk AML). A Cox Proportional Hazard model was used for OS analysis of the matched cohort.
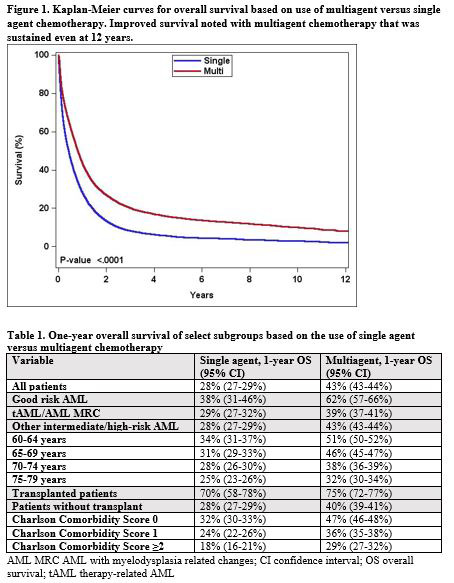
Results: Of a total of 25,621 patients, 70% received multiagent chemotherapy. The receipt of multiagent chemotherapy declined with increasing age, Charlson comorbidity score, AML subtypes other than good-risk, female, non-academic center, shorter distance traveled to receive care, lower rate of high school graduation, and more recent year of diagnosis. Patients treated with multiagent chemotherapy had higher likelihood of receiving hematopoietic cell transplant (HCT) (9% vs. 1%); lower one-month mortality (11% vs. 19%); and greater 1-year OS (43% vs. 28%) (Figure 1). The use of multiagent chemotherapy had particularly higher 1-year OS in patients aged 60-64 and 65-69 years, in good-risk AML, patients with Charlson comorbidity score of 0-1 and those who did not receive upfront HCT consolidation (Table 1). One-month mortality (odds ratio 1.64, 95% confidence interval, CI 1.51-1.78) and OS (hazard ratio, HR 1.32, 95% CI 1.28-1.36) remained more favorable for multiagent chemotherapy group in multivariable analyses. Other factors that affected OS included age, comorbidity, AML subtypes, median household income, insurance, use of HCT, academic status of facility, distance traveled to receive care, sex and year of diagnosis. In a matched analysis of 13,486 patients, the use of single agent vs. multiagent chemotherapy resulted in a higher risk of mortality (HR 1.28, 95% CI 1.23-1.32).
Conclusions: In one of the largest real-world studies, we demonstrate an association between factors such as age, comorbidity and AML subtypes and the use of multiagent chemotherapy. After adjusting for covariates, the use of multiagent chemotherapy among older adults was associated with higher receipt of HCT, and improved one-month mortality and OS. Improved OS was confirmed in a matched analysis. Certain groups such as patients younger than 70 years, good-risk AML and those with low Charlson comorbidity score had the greater OS benefit with the use of multiagent chemotherapy. Further studies to determine the role of multiagent or intensive chemotherapy are particularly important with approvals of several new drugs in the recent years and integration of many novel drugs in both low-intensity and intensive chemotherapy regimens.
Bhatt:Tolero Pharmaceuticals: Research Funding; Incyte: Consultancy, Research Funding; Partner therapeutics: Consultancy; Abbvie: Consultancy; Agios: Consultancy; CSL Behring: Consultancy; National Marrow Donor Program: Consultancy; Pfizer: Consultancy. Holstein:Celgene: Consultancy; Adaptive Biotechnologies: Membership on an entity's Board of Directors or advisory committees; GSK: Consultancy; Genentech: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Sorrento: Consultancy. Al-Kadhimi:Celldex Biotech: Other: Stocks; Seattle Genetics: Other: Stocks. Armitage:Union Pacific: Consultancy; Tesaro bio: Membership on an entity's Board of Directors or advisory committees; Ascentage: Consultancy; Samus Therapeutics: Consultancy; Oncology Analytics: Consultancy; Partner Therapeutics: Consultancy. Gundabolu:Pfizer: Consultancy; Novartis: Consultancy; Jazz pharmaceuticals: Consultancy; Samus Therapeutics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal