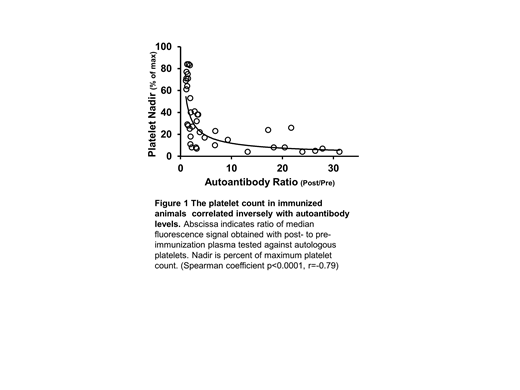
Post-transfusion purpura (PTP) is an uncommon but life-threatening condition characterized by profound thrombocytopenia (TP) occurring one week after transfusion of blood products. The hallmark of PTP is a potent IgG antibody specific for a transfused human platelet antigen (HPA), usually HPA-1a located on αIIb/β3 integrin (GPIIb/IIIa). It is widely thought that, in PTP, the alloantibody somehow causes destruction of the recipient's platelets even though they lack the antigen for which the alloantibody is specific. Several reports have suggested that the underlying cause of PTP is a platelet-specific autoantibody that can be difficult to detect because it is absorbed in the process of destroying autologous platelets and is overshadowed by the accompanying, very potent alloantibody but experimental support for this concept is minimal. Platelet alloantigens comparable to HPAs have not been defined in animals. Using a public database, we identified four mouse strains (C57BL/6J (C57), 129S1/Svlmj (129), PWK/PhJ (PWK), AND SPRET/EIJ) differing from each other at amino acid residues in extracellular domains of GPIIb/IIIa that could comprise potential alloantigens. Cross-strain platelet immunizations (intraperitoneal with adjuvant) were performed weekly for 5 weeks while monitoring platelet counts and platelet associated IgG (PAIgG) and saving plasma samples for serologic studies. After 2-4 immunizations, each of 39 cross-strain but none of 9 strain-identical immunizations induced "alloantibodies" that recognized donor but not recipient platelets (flow cytometry). Thrombocytopenia (<50% of maximum platelet count) developed in 28 of 39 mice (71%) given strain-disparate platelets but not in mice given strain-identical platelets; 12 of these mice (30%) developed profound TP (<15%). The most consistent and severe declines in platelet counts occurred in PWK mice immunized with 129 platelets and vice versa (N=13) in which the mean platelet count decline was 88% (range 59-96%, median 92%). Autoantibodies recognizing syngeneic platelets were identified in all animals that developed profound TP and their potency (measured by flow cytometry) correlated closely with the severity of TP (p<0.001) (Fig 1). Alloantibodies were shown by immunoprecipitation to be mainly specific for GPIIb/IIIa (N=13) and GPIb/IX (N=1) on donor platelets. Two monoclonal antibodies (mAbs MBC417.1 and MBC425.1) specific for a single polymorphic amino acid at positions 111(Gly) and 37(Val), respectively, on GPIIb of C57 and PWK mice were generated using spleen cells of two immunized mice. To our knowledge, these are the first alloantibodies in mice that are specific for single amino acid polymorphisms in a platelet membrane glycoprotein and are thus comparable to HPA antibodies found in humans.
The findings define a model (platelet immunization between PWK and 129 mice) in which a routine alloantibody response recognizing GPIIb/IIIa on donor platelets regularly transitions to an autoimmune response capable of causing profound thrombocytopenia, thus mimicking the course of PTP in human patients and supporting the hypothesis that PTP is an autoimmune disorder. Successful development of the model could be related to use of the more recently developed, wild-caught PWK strain as one of the partners for immunization. The inherent utility of a mouse model is expected to facilitate further work to define the molecular basis for a transition from allo- to auto-immunity in the human condition, post-transfusion purpura.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal