Background: Increased expression of hepatocyte growth factor (HGF), causing activation of its receptor MET, is found in a subset of patients with acute myeloid leukemia (AML). Inhibition of HGF-MET signaling with the specific MET kinase inhibitor crizotinib led to a transient therapeutic effect in AML cells; however, resistance rapidly emerged via increased HGF expression due to activation of alternative kinase pathways such as FGFR1 (Kentsis et al., Nat Medicine, 2012). Thus, simultaneous inhibition of activated MET and FGFR represents a potential therapeutic opportunity to forestall resistance to MET inhibition, in part by promoting downregulation of oncogenic STAT transcription factors. The MET/RON inhibitor merestinib and the pan-FGFR inhibitor LY2874455 are investigational small molecules. While they have been tested as single agents in phase 1 solid tumor cancer trials, they have neither been tested individually or in combination in AML patients (pts).
Methods: We are conducting a phase 1 combination study to determine the safety and tolerability of dose-escalated merestinib and LY2874455 in pts with R/R AML or secondary AML. Eligible pts include AML pts ≥ 18 y inappropriate for intensive chemotherapy, for whom no approved available therapy exists, and ECOG ≤ 2. A merestinib safety lead-in cohort (dose level (DL) 0) assessed merestinib alone (n=6) at a dose of 80 mg daily for a 28-day cycle (Cycle 0). In the absence of ≥ gr 3 adverse events (AEs), LY2874455 10 mg twice daily on days 1-21 was added to merestinib on day 1 of Cycle 1. Subsequent cohorts (DL 1-3) included a 7-day lead-in (day -6 through day 0) of merestinib alone (80 mg or 120 mg per assigned DL) for pharmacodynamic studies. DL1 is merestinib 80 mg daily for days 1-28 and LY2874455 10 mg po BID days 1-21. DL2 is merestinib 120 mg daily for days 1-28 and LY2874455 10 mg po BID days 1-21. DL3 is merestinib 120 mg daily for days 1-28 plus LY2874455 12 mg po BID days 1-28. Dose-limiting toxicity (DLT) is defined as non-hematologic AEs ≥ gr 3 (excluding fatigue or reversible electrolyte abnormalities), gr 4 infection, or gr 4 neutropenia > 42 days in absence of disease.
Results: The merestinib safety lead-in cohort consisted of 7 pts (2M, 5F) with a median age 76 y (range, 46-80) and a diagnosis of refractory (n=2) or relapsed (n=5) AML. One pt was replaced due to insufficient treatment doses administered. Four of 6 pts with available pre-treatment samples had adverse risk baseline mutations (TP53, RUNX1 and ASXL1) and 4 of 7 had poor risk cytogenetics including complex karyotype (n=3) and del 5q (n=1). Median duration on study was 2.4 months (90% CI, 0.8-5.6 months). AEs included 1 DLT due to gr 3 elevation of ALT and AST during merestinib monotherapy phase, which resolved with dose interruption and did not recur after dose modification. Other AEs were gr 3 bacteremia (n=1), gr 3 febrile neutropenia (n=1), gr 2 emesis (n=2), gr 2 nausea (n= 3), gr 3 diarrhea (n=1), gr 3 hypophosphatemia (n=2), gr 3 hyponatremia (n=1) and gr 3 QTc prolongation (n=1). SAEs included gr 4 ARDS (n=1), gr 3 back spasms (n=1), and gr 3 bruising (n=1), all considered to be unrelated to study drug.
Five of 6 evaluable pts had LY2874455 added to their treatment per protocol; 1 progressed at the end of merestinib-lead in. In this safety cohort, 1 achieved a CR (after 28 days of merestinib only), 4 had stable disease, and 1 had disease progression. The pt with a CR remained on merestinib monotherapy until progression at the end of cycle 4, at which point LY2874455 was added per protocol. Notably, this responder had baseline normal cytogenetics and mutations in DNMT3A (R882H), FLT3 (N676K), NPM1 (W288fs), TET2 (E227fs) and TET2 (L1231P). This activating FLT3 (N676K) mutation was not detected by repeat NGS with > 200X mean at remission.
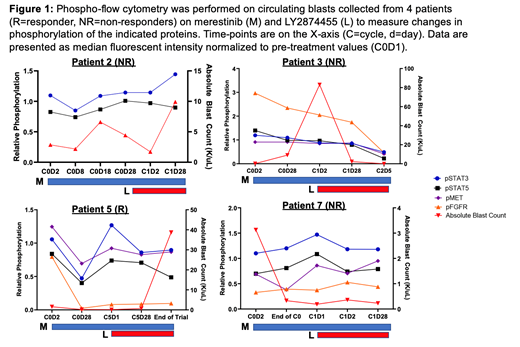
Exploration of the phosphorylation state of key signaling molecules (STAT3, STAT5, FGFR, and MET) potentially modified by merestinib and LY2874455 inhibitors was carried out in pts with circulating disease (Fig 1). The responder (pt 5) exhibited reduced signaling in pSTAT3, pSTAT5, pFGFR, and pMET during merestinib monotherapy concomitant with clearance of blasts, though this effect was lost shortly before relapse.
Conclusions: Preliminary clinical data suggest that merestinib is tolerable and the safety of adding dose-escalated LY2874455 is under investigation. Correlative studies to evaluate the significance of changes in HGF production and in STAT3/5 target genes are on-going.
Garcia:Abbvie: Research Funding; Genentech: Research Funding. Neuberg:Pharmacyclics: Research Funding; Madrigal Pharmaceuticals: Equity Ownership; Celgene: Research Funding. Winer:Jazz Pharmaceuticals, Pfizer: Consultancy. Galinsky:AbbVie Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Pfizer Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Merus Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; ABIM: Other: Member of specialty oncology board. DeAngelo:Glycomimetics: Research Funding; Blueprint: Consultancy, Research Funding; Amgen, Autolus, Celgene, Forty-seven, Incyte, Jazzs, Pfizer, Shire, Takeda: Consultancy; Abbvie: Research Funding; Novartis: Consultancy, Research Funding. Frank:Janpix, Roche-Genentech, Biolojib Design: Research Funding. Stone:Argenx, Celgene, Takeda Oncology: Other: Data and Safety Monitoring Board/Committee: ; Novartis, Agios, Arog: Research Funding; AbbVie, Actinium, Agios, Argenx, Arog, Astellas, AstraZeneca, Biolinerx, Celgene, Cornerstone Biopharma, Fujifilm, Jazz Pharmaceuticals, Amgen, Ono, Orsenix, Otsuka, Merck, Novartis, Pfizer, Sumitomo, Trovagene: Consultancy.
Merestinib and LY2874455 are investigational small molecular inhibitors that were tested in combination in a phase 1 clinical trial in AML.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal