Introduction
Novel high-risk groups have been identified in adult ALL, including secondary (sALL) and Philadelphia-like ALL (Ph-like, based on CRLF2, IgH, ABL2, JAK2 and other tyrosine kinase translocations), and those with minimal residual disease >0.1% (MRD+) after induction therapy. Novel targeted therapies are now routinely incorporated into 1st line regimens, including tyrosine kinase inhibitors (BCR-ABL1-pos), rituximab (CD20+) and blinatumomab (Blina) for MRD+. The impact of these novel high risk groups and therapies after alloHCT is unknown; therefore, we evaluated their impact on overall survival (OS), relapse rate (REL), non-relapse mortality (NRM) and acute and chronic GVHD.
Methods
We evaluated pts receiving 1st Allo-HCT for ALL at Mayo Clinic (Rochester, Phoenix, and Jacksonville) from 2008-2018 for outcomes of interest, specifically the impact of novel therapies and risk groups. Associations of patient factors with outcomes were examined using univariable (UVA) and multivariable (MVA) Cox proportional hazards regression models, where the cause-specific hazard of the given outcome was modeled to account for the competing risk of death.
Results
We identified 261 consecutive AlloHCT recipients during the study period. Median age at transplant was 48 years (18-72) and 147 (56.3%) were male. The median comorbidity (HCT-CI) score was 2 (0-8). 213 pts (81.6%) had B-lineage ALL, of which 85 (32.6%) were BCR-ABL-pos, 17 (6.5%) Ph-like (identified by FISH), 16 (6.1%) hypoploidy/near triploidy (Hy/Tri), and 67 (25.7%) pre-B ALL NOS. The remaining 48 (18.4%) had T-ALL. 30 pts (11.5%) had sALL (i.e. prior chemo/radiotherapy for another malignancy). HyperCVAD was the most common 1st line regimen (68.2%). 243 (93.1%) pts achieved Complete Remission (CR1) after induction therapy, and 203 (77.8%) were in CR1 at the time of alloHCT. Blina was administered for MRD+ in 14 pts (5.4%), and for relapsed/refractory ALL (R/R) in 13 (27% of R/R pts), 7 of whom received Blina as initial therapy for R/R.
Donors were matched unrelated in 149 (57.1%), matched related in 98 (37.5%), and haploidentical in 14 (5.4%). Peripheral blood (PB) grafts were used in 233 (89.3%). 103 (54.5%) were donor:recipient (D:R) sex-matched, and 86 D:R mismatched [47 (24.9%) M:F; and 39 (20.6%) F:M]. Myeloablative conditioning was used for the majority (78.5%) mostly with Cy/TBI (60.5%). Standard GVHD prophylaxis regimens were used.
Outcomes
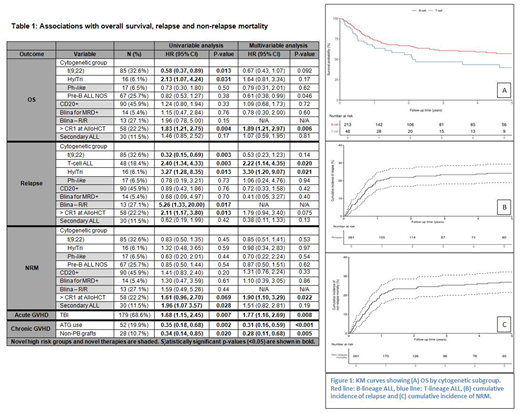
Median follow-up after transplant was 22.4 months (0.5-135), and 51 (19.5%) had REL. The 1, 2 and 5-year survival rates were 71.9%, 64.9%, and 54.1%, respectively (Figure 1). Acute GVHD developed in 144 (55.2%) and chronic GVHD in 100 (38.3%).
Ph-like ALL, Blina for MRD+, Blina for R/R, sALL and CD20-pos had no independent impact on OS. In contrast, age>60, Hy/Tri, and >CR1 at alloHCT were associated with worse OS in UVA, however, in MVA only pre-B ALL NOS was associated with better OS. Female:male D:R status was associated with inferior OS.
Blina for R/R disease was associated with increased risk of REL in UVA [HR 5.26 95% CI (1.33, 20.00), p=0.017], whereas other novel high risk groups had no impact on REL. In contrast, T-ALL, Hy/Tri and >CR1 at AlloHCT were associated with increased REL in UVA, but only T-ALL and Hy/Tri continued to predict for increased REL in MVA.
Secondary ALL was associated with increased NRM in UVA [HR 1.96 95% CI (1.07, 3.57), p=0.028], whereas other novel high risk groups had no impact on NRM. In contrast, age>60, >CR1 at AlloHCT and D:R sex mismatch were associated with higher NRM in UVA, but only sex mismatch and >CR1 at AlloHCT were associated with higher NRM in MVA.
TBI use was associated with higher risk of acute GvHD (p=0.008) and ATG use with lower risk chronic GVHD (p<0.001). Similarly non-PB grafts were associated with a lower risk of chronic GVHD (p=0.005).
Results for OS, REL, NRM, acute and chronic GVHD analysis are shown in Table 1.
Conclusion
Novel high risk groups (CD20+, Ph-like and sALL) do not appear to adversely impact OS after alloHCT, although sALL was associated with increased risk of NRM. Interestingly, pre-B-ALL NOS appear to be associated with favorable OS. Novel targeted therapies also do not independently predict outcome, with the exception of Blina for R/R ALL which may be associated with REL after subsequent alloHCT (a subgroup for whom novel maintenance strategies should be explored). Our analysis highlights the importance of allo-HCT for novel high risk ALL subgroups.
Patnaik:Stem Line Pharmaceuticals.: Membership on an entity's Board of Directors or advisory committees. Kharfan-Dabaja:Daiichi Sankyo: Consultancy; Pharmacyclics: Consultancy. Foran:Agios: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal