Background: DNA methyltransferase inhibitors sensitize leukemia cells to chemotherapeutics. Previous studies have indicated decitabine sequentially combined with idarubicin was an effective treatment for myeloid neoplasms. We therefore conducted a phase 2 study of combination of decitabine followed by low-dose idarubicin plus cytarabine in acute myeloid leukemia (AML) evolving from myelodysplastic syndrome (MDS) and higher risk MDS.
Methods: The study was a single-arm, prospective, multicenter study. Patients with AML evolving from MDS and refractory anemia with excess blasts (RAEB-2) high-risk MDS based on the 2008 WHO classification were included. The treatment plan consisted of decitabine 20 mg/m2 daily for 3 consecutive days on days 1-3, followed by idarubicin 6mg/m2 for 3 consecutive days on days 4-6, and cytarabine 25mg/m2 every 12 hours for 5 days on days 4-8. Granulocyte colony stimulating factor ( G-CSF) was given from day 4 and discontinued when neutrophil count increased to 1.0 × 109/L. In case remission was achieved after the first course, a similar second remission-induction course was given, to be followed by either an allogeneic hematopoietic stem cell transplantation (allo-HSCT) or subsequent consolidation courses, which was at the investigator's discretion.
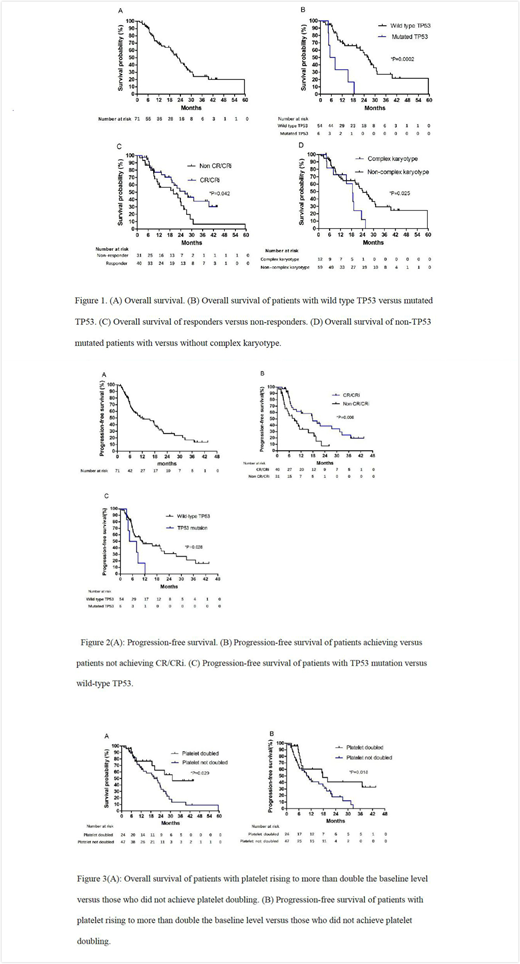
Results: A total of 71 patients were enrolled and treated, among whom 44 (62.0%) had AML evolving from MDS, 55 (77.5%) were previously untreated, 12 (16.9%) had an adverse karyotype, and 6 (10.0%) had TP53 mutations. 40 (56.3%) patients achieved a major response, including 20 (28.2%) patients with complete remission (CR) and 20 (28.2%) patients with complete remission with incomplete blood count recovery (CRi). The major response (CR+CRi) rate was 63.6% in AML patients and 44.4% in MDS patients (p=0.142). The median overall survival (OS) was 22.4 months (95% confidence interval (CI) ,17.8-27.0). The median OS was 24.2 months (95% CI 18.0-30.4) and 20.0 months (95% CI 11.2-28.7) for patients with AML and MDS (p=0.347), respectively. The median progress free survival (PFS) for the entire cohort was 12.1 months (95% CI 3.9-20.4). The median PFS was 17.6 months ( 95% CI 13.3-22.0) and 6.8 months (95% CI 0.1-13.6) for patients with AML and MDS (p=0.151), respectively. Patients who achieved CR/CRi had superior OS: 26.4 months ( 95% CI 17.6-35.2) vs. 20.0 months (95% CI 6.2-33.8) in non CR/CRi patients (p=0.042). Also patients who achieved CR/CRi had prolonged PFS: 17.8 months (95% CI 8.6-27.0) vs. 8.6 months (95% CI 3.5-13.8) in those not achieving CR. Patients with platelet doubling achieved significantly higher CR/CRi rate (24/24(100%) vs. 16/47(34.0%), p<0.01) and OR rate (24/24(100%) vs.36/47(76.6%), p=0.012) than those without platelet doubling. Also, patients with platelet doubling after the first cycle of therapy achieved prolonged OS (31.2 months with 95% CI not reached vs. 20.0 months with 95% CI of 15.0-25.0, p=0.029) and PFS (17.6 months with 95% CI of 3.4-31.9 vs. 10.6 months with 95% CI of 7.1-14.0, p=0.018) than patients without platelet doubling. The regimen was well tolerated, with one (1.4%) death within the first 6 weeks. Grade 3 or 4 hematological toxicities were reported in all of the cases. The hematopoietic recovery of patients who achieved CR/CRi was shown by the median recovery of neutrophils to 0.5×109/L of 21 days (range:8-53) and of platelets to >50×109/L of 25 days (range: 8-41).
Conclusions: The regimen of decitabine priming followed by low-dose idarubicin plus cytarabine appears to be highly effective and has an acceptable safety profile in patients with AML evolving from MDS or RAEB-2. Further controlled studies are needed to confirm the activity of this regimen, and could help to provide an alternative option for induction therapy in this population.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal