BACKGROUND
Secondary AML (sAML) that develops after an antecedent hematologic disorder or exposure to genotoxic therapy is associated with a poor prognosis and historical long-term survival rates of 5-10% (Granfeldt Østgård et al. 2015). Liposomal daunorubicin and cytarabine (CPX-351) was recently FDA approved for the treatment of sAML, based on a randomized phase III trial that found a higher complete remission (CR) rate, event-free survival (EFS), and overall survival (OS) with CPX-351 compared to 7+3 chemotherapy (Lancet et al. 2018). Retrospective studies conducted in this patient population suggest that high-dose cytarabine (HIDAC)-based regimens may also be safe and effective in sAML (Vulaj et al. 2018, Talati et al. 2018). With an average wholesale price of up to $150,000 for induction, CPX-351 represents a large financial burden when compared to HIDAC-based regimens. Because HIDAC-based regimens employ older, generic medications, and sAML is a relatively rare diagnosis, it is unlikely these two induction strategies will be compared prospectively. This real-world, multicenter study retrospectively compared clinical outcomes in patients with sAML receiving CPX-351 and HIDAC-based regimens.
METHODS
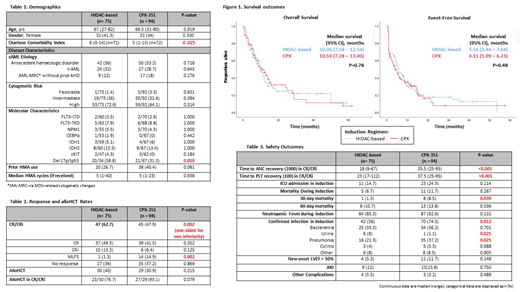
Adult patients with newly diagnosed sAML treated between January 2013 and January 2019 from 7 academic medical centers (University of Michigan n=73, MD Anderson Cancer Center n=27, Barnes Jewish Hospital n=22, University of North Carolina n=21, Huntsman Cancer Institute n=9, University of Rochester n=9, Indiana University n=8) were divided into 2 cohorts based on induction regimen: HIDAC-based or CPX-351. Demographics, disease characteristics, and outcomes were collected in accordance with the institutional review board approved protocol. The primary endpoint of composite complete response/complete response with incomplete count recovery (CR/CRi) as well as secondary efficacy endpoints were defined using 2003 International Working Group Criteria. For the primary endpoint, a sample of 96 patients was established a priori as necessary to demonstrate non-inferiority of HIDAC-based regimens using a non-inferiority margin of 7.5% and historical CR/CRi rates or 65% and 47.7% for HIDAC-based and CPX-351 respectively. Chi-square or Fisher's exact tests were utilized to evaluate dichotomous variables. Continuous variables were analyzed via Student's t-test or Mann-Whitney U test. Kaplan-Meier analysis with log-rank test was performed to estimate progression-free survival (PFS) and overall survival (OS).
RESULTS
A total of 169 patients were included (HIDAC-based: n=75; CPX-351: n=94). HIDAC-based regimen distribution was as follows: fludarabine/cytarabine ± G-CSF (n=73) and clofarabine/cytarabine ± G-CSF (n=2). Baseline characteristics were well balanced (table 1) with the exception of a higher Charlson Comorbidity Index in patients who received HIDAC-based therapy. The median age of all patients was 67 years and the majority of patients had sAML due to an antecedent hematologic disorder. CR/CRi rate was numerically higher and non-inferior with HIDAC-based therapy (62.7%) compared with CPX-351 (47.9%) (p=0.002 [one-sided for non-inferiority]). Median OS was 10.06 vs 10.59 months and median EFS was 5.56 vs 4.11 months with HIDAC-based regimens compared with CPX-351, respectively. Thirty-day mortality (1.3 vs 8.5%; p=0.039) and confirmed infection in induction (56% vs 74.5%; p=0.012) were significantly higher with CPX-351. Additionally, the time to absolute neutrophil count (ANC) and platelet count (PLT) recovery in CR/CRi were significantly longer with CPX (ANC: 18 vs 35.5 days; p<0.001; PLT: 23 vs 37.5 days; p<0.001).
CONCLUSIONS
HIDAC-based regimens yield CR/CRi rates that are non-inferior to CPX-351 in patients with sAML with similar EFS and OS. In a primarily elderly population, HIDAC-based therapy demonstrates greater tolerability with lower induction mortality, infection rates, and a more favorable duration of cytopenias. With non-inferior efficacy, better tolerability, and significantly lower cost, HIDAC-based regimens may be preferred to CPX-351 in patients with sAML.
Klaus:Jazz Pharmaceuticals: Other: Advisory Board, Speakers Bureau. Clark:Elliott Benson Research: Consultancy. Pettit:Samus Therapeutics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal