Introduction
One of the contributing factors to the relapse of acute myeloid leukemia (AML) is the presence of leukemia stem cells (LSCs). Interleukin 1 receptor accessory protein (IL1RAP) was reported to be one of the LSC markers. Most studies regarding clinical implications of IL1RAP expression in AML focused on small and selected patient groups. Besides, its correlation with other molecular alterations has not been reported yet in literature. In this study, we aimed to elucidate the relationship between bone marrow IL1RAP expression level and clinical and biological features in patients with de novo non-M3 AML. Furthermore, we would like to explore its prognostic impact and potential underlying mechanism.
Method
We enrolled 275 newly diagnosed de novo non-M3 AML patients. Among them, 187 (68%) patients received standard induction chemotherapy and 2-4 courses of high-dose cytarabine based post-remission therapy. Analyses of 54 gene mutations were performed by next generation sequencing. The global gene expressions were profiled with the Affymetrix GeneChip Human Transcriptome Array 2.0.
Result
We used the median as the cut-off value to define the higher and lower IL1RAP expression groups. The patients with higher IL1RAP expression had significantly higher white blood cell counts at diagnosis, higher peripheral blast counts, and higher lactate dehydrogenase levels. Higher IL1RAP expression was closely associated with t(8;21), favorable-risk cytogenetics based on the refined MRC classification, but inversely with unfavorable-risk cytogenetics. Compared with low-expression patients, the high-expression patients had significantly more FLT3/ITD and KIT mutations, but less mutations in U2AF1, TP53, or CEBPA.
Among the 187 patients receiving standard intensive chemotherapy, those with lower IL1RAP expression had significantly longer overall survival (OS) than those with higher expression (P=0.047) after a median follow-up time of 91.1 months, but disease-free survival (DFS) was not significantly different between the two groups (P=0.311). Among the 77 patients who relapsed after first complete remission (CR), the second CR rate was similar between the two groups (P=0.649), but the second DFS was significantly longer in the low-expression patients than the high-expression patients (P=0.028) which was also reflected in a significantly longer survival after first relapse in the former group than the latter group (P=0.014). The prognostic impact of IL1RAP expression on OS could be externally validated in the TCGA cohort (P=0.038). Its prognostic implication remained significant in the subgroup of our cohort with intermediate-risk cytogenetics (P=0.006) and those with normal karyotype (P=0.025). In multivariate analysis incorporating age, transplantation status, 2017 ELN risk-stratification and IL1RAP expression as covariates, the higher IL1RAP expression was an independent poor prognostic factor for OS (HR=1.555, P=0.025).
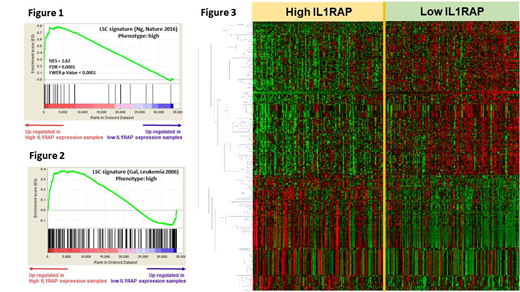
The Gene Set Enrichment Analysis revealed significant up-regulation of LSC related genes in the higher IL1RAP expressed patients (Figure 1 and 2). We further profiled genome-wide RNA expression with 70,523 probes to survey the potential molecular mechanisms underlying the IL1RAP expression signature. Totally, 313 differentially expressed genes were identified (>1.5-fold change and Student t-test P<0.0001, Figure 3). We used Ingenuity Pathway Analysis (Qiagen) to analyze the possible underlying mechanism and found that the top upstream regulators were transcription factors, such as GATA1/GATA2 (P=1.39*10-11 and 1.61*10-10, respectively), and ABCB6 (P=3.84*10-8), one of the ATP-Binding Cassette transporter superfamily. The hub genes in the regulation network included ELAVL1 and NFκB, in addition to GATA1 and GATA2.
Conclusion
Higher IL1RAP expression is associated with distinct clinical and genetic alterations. It is an independent prognostic factor for OS irrespective of the risk category based on the ELN classification. Transcription factors, such as GATA1 and GATA2, ABCB6, ELAVL1 and NFκB might be involved in the underlying mechanism. Further prospective large cohort is warrant to validate our findings.
Tien:Novartis: Other: Travel Grant. Hou:Celgene: Research Funding; Abbvie, Astellas, BMS, Celgene, Chugai, Daiichi Sankyo, IQVIA, Johnson & Johnson, Kirin, Merck Sharp & Dohme, Novartis, Pfizer, PharmaEssential, Roche, Takeda: Honoraria. Tien:Daiichi Sankyo: Honoraria; Roche: Honoraria; Abbvie: Honoraria; Alexion: Honoraria; Celgene: Honoraria; Johnson &Johnson: Honoraria; Novartis: Honoraria; Celgene: Research Funding; BMS: Honoraria; Pfizer: Honoraria; Roche: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal