Background: Tyrosine kinase inhibitors (TKIs) are able to induce, in some chronic myeloid leukemia (CML) patients in chronic phase (CP), long-term molecular response 4.5 (MR4.5) and several studies have now demonstrated that TKIs could be safely discontinued in those patients with a Treatment-Free Remission (TFR) rate reaching ~50%. The French CML group had recently demonstrated that a failure of the first TKI discontinuation attempt does not preclude a 2nd successful attempt (RE-STIM study, Legros et al. Cancer 2017).
Methods: The RE-STIM study is a national observational multicentre study collecting all cases of 2nd TKI discontinuation attempt of regardless the type, the duration of TKI, the duration of MR4.5 and the reason of discontinuation. CP-CML Patients in failure of a 1st attempt, had to recover a 2nd sustained MR4.5 on TKI to be eligible for this new analysis of the enlarged database (n=106). Loss of MMR loss was the trigger for therapy re-introduction.
Results: At the time of analysis (1st June 2019), 106 patients (median age: 55 years (range: 25-81 years)) were included with 41 months (2-131) of follow-up after 2nd discontinuation. Fifty males and 56 females were enrolled. The Sokal risk score was low in 45%, intermediate in 26.5%, high in 20% and unknown in 8.5% of patients. The majority of patients (95%) were treated with imatinib as first-line, and the others with a 2nd generation TKI. The median total time on TKI prior to a 2nd discontinuation was 104 months (range: 38-235) and the median duration of a 2nd MR4.5 prior to a 2nd discontinuation was 68 months (range: 20-176). After a 1st discontinuation attempt, the reason for TKI re-challenge was in majority a loss of MMR (66%), a loss of MR4.5 in 33% of patients (missing data in 1%).
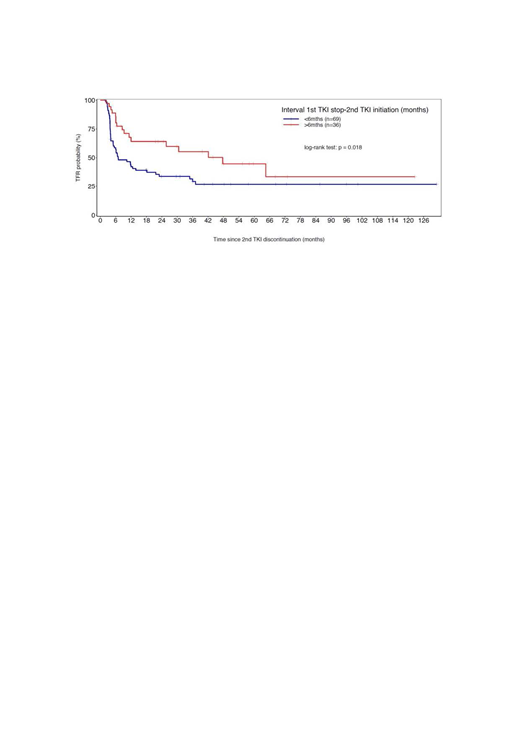
The TFR rates after a 2nd discontinuation attempt were 44.3% [95% CI 35.48-55.41] at 24 months, 38.5% [95% CI 29.65- 50.09] at 36 months and 33.2% [95% CI 24.31- 45.39] at 48 months. In univariate analysis, we failed to find any association between TFR and: age, gender, Sokal score, prior exposure to IFN, TKI in combination versus monotherapy, TKI type, TKI treatment duration and uMR4.5 duration before the 1st and 2nd discontinuation attempts, and type of molecular relapse after the 1st discontinuation attempt (MR4.5 versus MMR loss). However, the speed of molecular relapse after the 1st TKI discontinuation remains a factor significantly associated with outcome. In patients who remained in uMR4.5 at 3 months after the 1st discontinuation, the TFR rate at 48 months was 53% [95% CI: 35.32-79.31] and 26% [95% CI: 16.88-40.28] for others. Another factor significantly associated with outcome is the TKI-free duration after the 1st attempt (Figure). The TFR rate at 48 months was 45 % [95% CI: 28.64- 69.62] in patients who remained without treatment more than 6 months after their 1st attempt and 27% [95% CI: 17.57- 41.34] for others.
All patients are alive at last follow-up except 2 who died from CML-unrelated reasons. One patient developed a sudden blast crisis at 4 years from 2nd discontinuation. The last previous molecular biology 3 months before transformation was MR4. In patients in TKI re-challenge (n=63), median TKI-free duration was 6 months (2-64), 55% of patients regained their MMR within 3 months (0-35) and 41% regained MR4.5 within 5 months (2-53).
Conclusions: This larger cohort confirms that TKIs could safely and successfully be discontinued a 2nd time in CP CML patients despite a 1st failure. The speed of molecular relapse after the 1st TKI discontinuation and TKI-free duration remain major factors significantly associated with TFR outcome.
Figure: TFR according TKI-free duration after the 1st attempt of discontinuation
Legros:Incyte Biosciences: Honoraria, Research Funding; BMS: Honoraria; Pfizer: Honoraria, Research Funding; Novartis: Honoraria. Nicolini:Sun Pharma Ltd: Consultancy; Incyte Biosciences: Honoraria, Research Funding, Speakers Bureau; Novartis: Research Funding, Speakers Bureau. Etienne:BMS: Honoraria, Speakers Bureau; Incyte Biosciences: Honoraria, Speakers Bureau; Pfizer: Honoraria, Speakers Bureau; Novartis: Consultancy, Honoraria, Speakers Bureau. Rousselot:Pfizer: Research Funding; Incyte: Research Funding. Rea:Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria; Incyte Biosciences: Honoraria. Guerci:INCYTE: Consultancy, Honoraria. Huguet:Incyte Biosciences: Honoraria; Novartis: Honoraria; Servier: Honoraria; Jazz Pharmaceuticals: Honoraria; Amgen: Honoraria; BMS: Honoraria; Pfizer: Honoraria. Coiteux:Novartis: Honoraria, Speakers Bureau; BMS: Honoraria, Speakers Bureau; Incyte Biosciences: Honoraria, Speakers Bureau; Pfizer: Honoraria, Speakers Bureau. Mahon:Novartis: Consultancy, Honoraria, Speakers Bureau; BMS: Honoraria, Speakers Bureau; Pfizer: Honoraria, Speakers Bureau; Incyte Biosciences: Honoraria, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal