Mantle cell lymphoma (MCL) is one subtype of the B cell non-Hodgkin lymphomas (NHL) and has a distinguash course. Despite all efforts, MCL is nearly incurable that refractory or relapse(r/r) course will occur in almost all patients. Due to the rapid development of chemotherapy resistance, it is difficult to treat r/r MCL. A number of treatment regimens have been evaluated with various degrees of success. Cellular immunotherapy has the potential to lead significant and sustained clinical remissions in lymphoma patients, in which modification of T cells with chimeric antigen receptors (CARs) is a powerful regimen. T cells containing CARs with costimulatory domains show better activity against tumor cells. We conducted a clinical trial testing a "second-generation" CD19-specific CAR with 4-1BB costimulatory domains in patients with r/r MCL.
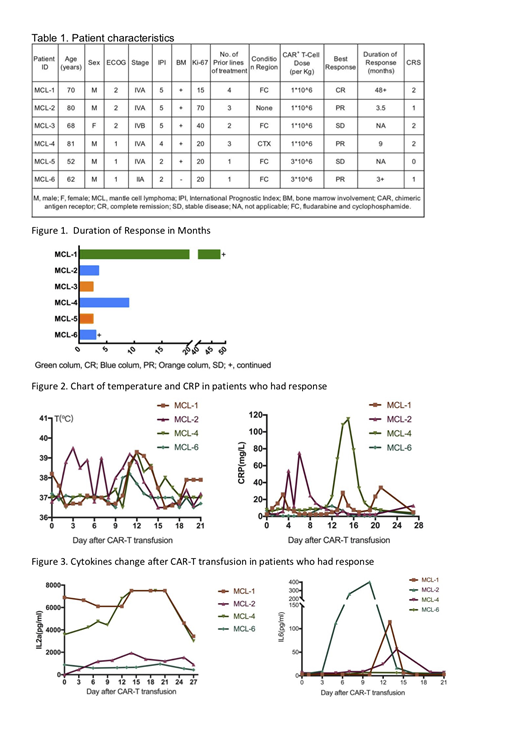
Six patients with r/r MCL were enrolled and all were received T-cell infusions. The age of patients ranged from 52 to 81 years old. Five patients were male and one patient was female. Five patients had stage IV MCL with bone marrow involvement and one patient was stage II. Four patients had received more than 3 lines regimens and two patients had received first-line chemotherapy regimen. Four patients received FC regimen before CAR-T infusion and two patients were not received fludarabine due to age ≥80 years old.
Treatment was well tolerated, two patients developed transient infusion reaction. No patients have grade 3-4 Cytokine Release Syndrome (CRS). Three patients have grade 2 CRS and two patients have grade 1 CRS. Objective response rate is 66.67%. One patient achieves complete remission(CR) and three patients have partial remission(PR). The response of the other two patients is stable disease(SD). The patient who has CR response has remained progression-free for 36 months and is still being followed up. Two patients who have PR response have progressed in 3 months and the other one has durable response for 7 months. In the patients who has response, the peak of fever appears at approximately day 10 after CAR-T infusion, moreover interleukin(IL)6,IL2αand interferon(IFN)-γ almost reaches the peak on the day 14.
In conclusion, adoptive immunotherapy with CD19-specific T cells was well tolerated and was associated with antitumor activity. Further large-sample and multicenter studies are needed to fully evaluate this strategy for such patients.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal