Background: Aggressive B-cell lymphomas that secondarily involve the CNS (sCNSL) have a grave prognosis and no standard of care. Treatment includes chemotherapy that penetrates the CNS, such as high-dose methotrexate, but are relatively ineffective for extracranial disease. Diffuse large B-cell lymphomas with secondary CNS involvement are enriched for chronic active B-cell receptor signaling, and are responsive to BTK inhibitors. Ibrutinib is also active in other B-cell malignancies that spread to the CNS including MCL and transformed lymphomas. Ibrutinib achieves CNS penetrance and has a high overall response rate in CNS lymphomas, but duration of response is short and curative potential is limited (Grommes et al. 2017). Further, toxicities associated with ibrutinib can compromise outcomes when added to chemotherapy. Precise understanding of molecular determinants of response could enhance patient selection strategies.
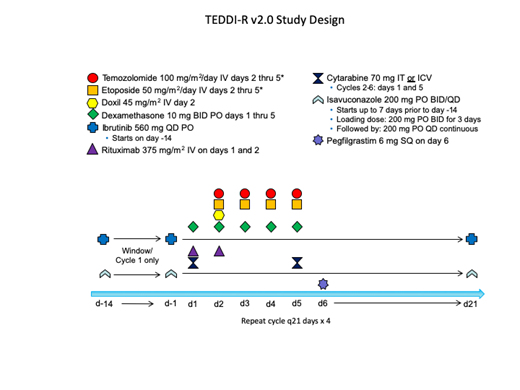
We developed a novel regimen that combines ibrutinib with temozolomide, etoposide, doxil, dexamethasone, and rituximab (TEDDi-R) for relapsed and refractory PCNSL. TEDDi-R achieved durable remissions in chemotherapy-refractory PCNSL (Roschewski et al. ASH 2018), but has been associated with opportunistic infections, including Aspergillus. Development of TEDDi-R continues with isavuconazole prophylaxis that inhibits clearance of ibrutinib. We opened a novel response-adapted clinical trial designed to study the safety and efficacy of TEDDi-R in sCNSL and elucidate molecular correlates of response to ibrutinib.
Study Design and Methods:
Phase II study of 29 evaluable patients with recurrent sCNSL [NCT03964090]
Patients with untreated aggressive B-cell lymphomas are eligible if CNS lesions involve brain parenchyma
Patients are first treated with a 14-day "window" of ibrutinib 560mg monotherapy with concomitant isavuconazole
Response adapted: Patients with at least a 20% reduction in bidimensional masses following the 14-day ibrutinib window will receive ibrutinib with chemotherapy (TEDDi-R) for 4 cycles. Patients with less than a 20% reduction during the ibrutinib window will receive TEDD-R (without ibrutinib) for 4 cycles
Primary endpoint: progression free survival (PFS)
Secondary endpoints:
Safety of TEDDi-R and TEDD-R
Clinical activity after 14 days of ibrutinib monotherapy
Best overall response rate of 4 cycles of TEDDi-R/TEDD-R
Duration of response
PKs and safety of ibrutinib and TEDDi-R with concomitant isovuconazole prophylaxis
Overall survival
Translational endpoints:
Comprehensive molecular analysis of tumors using gene expression profiling, whole exome sequencing, and RNAseq analysis correlated with outcome
Genotype cell-free DNA in plasma and CSF correlated with clinical response
Eligibility:
Included
Age ≥ 18
Prior BTK inhibitors permitted, but patients known refractory to BTK will proceed directly with TEDD-R
Patients with CLL or FL that have progressed in the CNS are considered to have transformed to an aggressive B-cell lymphoma and eligible
ECOG performance status ≤2
Adequate organ function
Willing to take appropriate contraception
Excluded
HIV
Inability to take isavuconazole
Statistical methods: Expected median PFS would be less than 4 months based on historical data. The goal is to improve to a median 10 months PFS. With 29 evaluable patients, there would be 90% power to determine a difference between a 4-month median PFS probability and an improved 10-month median PFS, with a one sided 0.05 alpha level test, using the method of Brookmeyer and Crowley. Confidence intervals and PFS probabilities will be reported, but no formal hypothesis test will be undertaken.
Summary:
This is a phase 2 study of TEDDi-R in aggressive B-cell lymphomas with secondary involvement of CNS that employs a novel response-adapted design for ibrutinib. The aim is to enhance selection of patients likely to benefit from ibrutinib and to investigate the molecular determinants of response to BTK inhibition. The study is open and the first patient has been enrolled. All patients will be treated at the NCI, but the development of TEDDi-R is supported by a consortium of regional academic centers who provide academic input, patient referrals, and data analysis. Current participating centers include Johns Hopkins University, George Washington University, University of Pennsylvania, University of Virginia, University of Pittsburgh and Penn State University.
Dunleavy:Pharmacyclics: Membership on an entity's Board of Directors or advisory committees. Swinnen:Pharmacyclics: Consultancy; AbbVie: Consultancy. Portell:Kite: Consultancy, Research Funding; Infinity: Research Funding; Roche/Genentech: Research Funding; Acerta/AstraZeneca: Research Funding; TG Therapeutics: Research Funding; Xencor: Research Funding; AbbVie: Research Funding; Pharmacyclics: Consultancy; Janssen: Consultancy; Genentech: Consultancy, Research Funding; Amgen: Consultancy; Bayer: Consultancy; BeiGene: Consultancy, Research Funding. Svoboda:Pharmacyclics: Consultancy, Research Funding; AstraZeneca: Consultancy; Seattle Genetics: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Merck: Research Funding; Incyte: Research Funding; Celgene: Research Funding; Kite: Consultancy; Kyowa: Consultancy. Staudt:Nanostring: Patents & Royalties.
Ibrutinib used off-label for CNS lymphomas
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal