Background: Myelofibrosis (MF) is a myeloproliferative neoplasm (MPN) characterized by progressive bone marrow fibrosis, ineffective erythropoiesis, dysplastic megakaryocyte hyperplasia, and extramedullary hematopoiesis. MF includes primary MF (PMF), post-polycythemia vera MF (post-PV-MF), and post-essential thrombocythemia MF (post-ET-MF). Clinical presentation is heterogeneous, marked by splenomegaly, progressive anemia, and constitutional symptoms. The median survival in patients with high-risk disease is approximately 2 years. Hematopoietic Stem Cell Transplant (HSCT) is a potentially curative therapy, however due to considerable morbidity and mortality rates, HSCT is not appropriate for most patients, including elderly patients with intermediate-II and high-risk disease. The Janus kinase (JAK) inhibitor ruxolitinib is approved in the US and EU for the treatment of patients with intermediate or high-risk MF, including PMF, post-PV-MF, and post-ET-MF. In clinical studies, treatment with ruxolitinib has been shown to reduce spleen volume by International Working Group (IWG) criteria in approximately 28% to 42% of patients and improve constitutional symptoms of MF in approximately 46% of patients (Verstovsek, J Hematol Oncol. 2017). Ruxolitinib provides symptomatic improvement, however, does not target the malignant clone or appreciably reduce the degree of fibrosis; some patients experience disease progression and leukemic transformation while on therapy (Versotvsek, NEJM. 2010; Harrison, NEJM. 2012; Kremyanskaya, Br J Hem. 2014). Moreover, ruxolitinib is associated with AEs including anemia and thrombocytopenia, which can lead to discontinuation. Approximately 50% of patients treated with ruxolitinib discontinued treatment within 3 years and 73% at 5 years (Verstovsek, Haematologica. 2015; Verstovsek, J Hematol Oncol. 2017; Cervantes, Blood. 2013; Harrison, Leukemia. 2016). Median overall survival in patients who discontinue ruxolitinib is 14-16 months, highlighting the need for novel therapies targeting alternative pathways in the setting of failure or intolerance of JAK inhibitor therapy (Newberry, Blood. 2017).
The tumor suppressor protein p53 is the master regulator of cell-cycle arrest and apoptosis in response to cellular stress or DNA damage. Murine double minute 2 (MDM2) is a key regulator of p53, inhibiting its activity via ubiquitination, nuclear export, and direct inhibition of transcriptional activity. Increased MDM2 protein expression has been observed in MF CD34+ cells, suggesting that MF might be sensitive to MDM2 inhibition (Lu M, Blood. 2017). KRT-232 is a potent and selective, oral, small molecule drug that targets MDM2 and prevents MDM2-mediated p53 inhibition, allowing p53 to mediate tumor cell-cycle arrest and apoptosis. In MF, TP53 is observed to be wild-type in 96% of MF patients, suggesting MDM2 inhibition could be a successful therapeutic strategy in this disease (Raza, Am J Hematol. 2012). KRT-232 has been investigated as monotherapy and in combination with trametinib or dabrafenib in phase I studies of AML and melanoma; the most common treatment-related adverse events (TRAEs) observed were nausea, diarrhea, vomiting, decreased appetite, anemia, leukopenia, thrombocytopenia, and fatigue. The majority of TRAEs were grade 1 or 2.
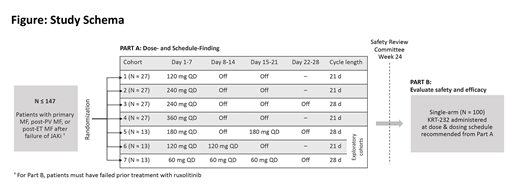
Methods: KRT-232 is being evaluated in an open-label phase 2 study in patients with MF who relapsed on or are refractory to JAK inhibitors (Figure). Up to 247 patients ≥ 18 years of age, with ECOG performance status ≤ 2, with high-, intermediate-2, or intermediate-1 risk disease by Dynamic International Prognostic System (DIPSS), and failure of prior treatment with JAK inhibitors will be enrolled. The study will be conducted in 2 parts. Part A will identify the recommended dose and schedule by testing varying doses and schedules across 7 treatment cohorts. Part B will evaluate safety and efficacy using the recommended dose and schedule from Part A. The primary endpoint of the study is to determine spleen response at week 24; secondary endpoints include improvement in MPN-SAF Total Symptom Score (weeks 24 and 48), red blood cell (RBC) transfusion independence, and rates of complete remission and partial remission (IWG-ERT and ELN) at week 24. This trial is enrolling at multiple sites in the United States and Europe (NCT03662126, EudraCT: 2018-001671-21).
Garcia-Delgado:Hospital Virgen De La Victoria Malaga: Employment; Novartis: Consultancy, Speakers Bureau; Celgene: Speakers Bureau. McLornan:Jazz Pharmaceuticals: Honoraria, Speakers Bureau; Novartis: Honoraria. Jourdan:Novartis: Honoraria; Astellas: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees. Al-Ali:Celgene: Research Funding; Novartis: Consultancy, Honoraria, Research Funding; CTI: Honoraria. Pluta:Freelight Poland: Honoraria; Sandoz: Honoraria; Servier: Honoraria; Jansen-Cilag: Honoraria; Novartis: Honoraria; Takeda: Honoraria; Roche: Honoraria; Specialistic Hospital in Brzozow,Dept of Haematooncology Ks.Bielawskiego 18 36-200 Brzozow, Poland: Employment; Teva: Honoraria; Roche Poland: Membership on an entity's Board of Directors or advisory committees; Jansen Cilag Poland: Membership on an entity's Board of Directors or advisory committees. Ewing:Novartis: Honoraria, Other: Meeting attendance sponsorship ; Bristol Myers-Squibb: Other: Meeting attendance sponsorship . Khan:Amgen: Consultancy; Celgene: Consultancy; Incyte: Honoraria; Pfizer: Consultancy; Takeda: Research Funding. Jost:Novartis: Research Funding; Celgene: Other: Travel Support; Pfizer: Consultancy, Speakers Bureau; Novartis: Consultancy, Speakers Bureau; Abbvie: Consultancy, Patents & Royalties: Royalty payments for the drug compound ABT-199, Research Funding; Bohringer: Consultancy, Research Funding; BMS: Consultancy, Speakers Bureau. Rothbaum:Kartos Therapeutics: Employment, Patents & Royalties: Pending; Quogue Bioventures LLC: Equity Ownership, Membership on an entity's Board of Directors or advisory committees. McGreivy:Kartos Therapeutics: Employment, Equity Ownership, Membership on an entity's Board of Directors or advisory committees. Verstovsek:Incyte: Research Funding; Roche: Research Funding; NS Pharma: Research Funding; Celgene: Consultancy, Research Funding; Gilead: Research Funding; Promedior: Research Funding; CTI BioPharma Corp: Research Funding; Genetech: Research Funding; Blueprint Medicines Corp: Research Funding; Novartis: Consultancy, Research Funding; Sierra Oncology: Research Funding; Pharma Essentia: Research Funding; Astrazeneca: Research Funding; Ital Pharma: Research Funding; Protaganist Therapeutics: Research Funding; Constellation: Consultancy; Pragmatist: Consultancy.
Yes, KRT-232 is an investigational small molecule MDM2 inhibitor. This trial-in-progress abstract describes a registered clinical trial that will evaluate the safety and efficacy of KRT-232 for patients with myelofibrosis.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal