Introduction. Genetic studies in patients with Ph-negative myeloproliferative neoplasms (MPNs) are essential to establish a correct diagnosis and to optimize their management. Recently, it has been demonstrated that it is possible to detect molecular alterations present in solid tumors and hematologic neoplasms by the analysis of circulating tumor DNA in plasma samples, which is known as liquid biopsy. It has been reported that most of the circulating cell-free DNA (cfDNA) has its origin in immature hematopoietic and bone marrow cells; however, there is limited information about liquid biopsy applications in MPNs.
Objective. To analyze the molecular profile of circulating tumor DNA in patients with MPNs.
Patients and methods. Peripheral blood samples from 75 patients with MPNs were collected at the time of diagnosis: 21 polycythemia vera (PV), 42 essential thrombocythemia (ET), 10 primary myelofibrosis (PMF) and two non-classifiable MPNs. Cellular DNA was extracted from the granulocytic fraction isolated by density gradient centrifugation and cfDNA was obtained from 1-3ml of plasma (MagMAX Cell-Free DNA Isolation Kit, Thermo Fisher Scientific). cfDNA purity was ascertained by capillary electrophoresis (4200 TapeStation system, Agilent). Molecular characterization was performed in paired samples of granulocytes DNA and cfDNA by next generation sequencing (NGS). Libraries were prepared using a custom panel that covered the whole codifying region of 25 myeloid-associated genes (QIAseq Custom DNA Panels, Qiagen) and sequenced using Illumina technology (Miseq, Nextseq) with a 3000x minimum coverage.
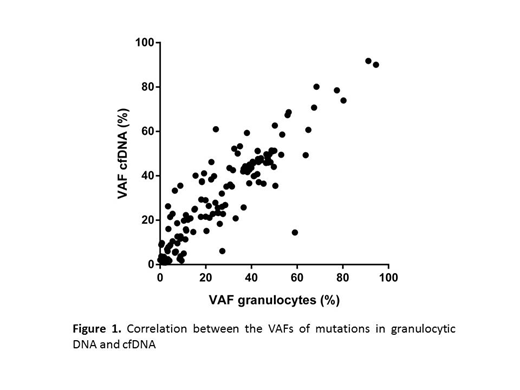
Results. The amount of total cfDNA/mL in plasma was significantly higher in PMF (mean 97 ng/ml) than in PV and ET (mean 18 and 23g/ml, respectively) (p = 0.003, Kruskal-Wallis). Overall, 144 mutations in driver (JAK2, CALR, MPL) and non-driver genes were detected in the granulocytic fraction with similar frequencies to what has been described for PV, ET and PMF. The most frequently mutated non-driver genes where ASXL1 (18.7%), TET2 (17.3%), DNMT3A (6.7%), SRSF2 (6.7%) and IDH2 (5.3%). Sequencing of cfDNA showed a total of 146 mutations. All mutations detected in the granulocytic fraction were also detected in the paired cfDNA sample (100% concordance); two additional mutations in MPL and ASXL1 were detected in plasma in one case. The median variant allele frequency (VAF) present in cfDNA was 29% (range 0.86 - 91.73%), which is far superior to what has been described in solid neoplasms or lymphomas (median 0.41%, range 0.03% - 97.6%). A strong correlation was observed between the VAFs of granulocytic DNA and cfDNA (r = 0.875, p < 0.001, Spearman) (Figure 1). The mutation VAFs detected in cfDNA were significantly higher than VAFs detected in granulocytes (p < 0.001, Wilcoxon). In particular, MPL mutations presented 2.5 higher VAF in cfDNA than in granulocytes (p = 0.018, Wilcoxon). This finding was confirmed and quantified by digital PCR. Interestingly, in one PMF patient the p.W515L MPL driver mutation was originally only detectable by NGS in cfDNA, but not in granulocytes. This mutation was identified by ultra-sensitive digital PCR in both cfDNA (VAF 2.30%) and granulocytes (VAF 0.16%).
Conclusions. The analysis of circulating tumor DNA allows the characterization of the molecular abnormalities of patients with Ph negative myeloproliferative neoplasms. The sensitivity for mutation detection in driver and non-driver genes was equal or even superior to that obtained when studying the isolated granulocytic population.
Salar:Roche: Research Funding, Speakers Bureau; Janssen Pharmaceuticals: Consultancy, Speakers Bureau; Gilead: Consultancy, Speakers Bureau; Celgene: Consultancy. Besses:Gilead: Research Funding. Bellosillo:TermoFisher Scientific: Consultancy, Speakers Bureau; Qiagen: Consultancy, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal