BACKGROUND: Minimal residual disease (MRD) negativity is one of the strongest predictors of outcome after first-line treatment with chemoimmunotherapy. Likewise, persisting MRD-positivity defines patients (pts) with chronic lymphocytic leukemia (CLL) at a high risk of short overall survival (OS). A cross‐trial comparison of randomized, phase 3 data allows an OS analysis of high risk pts over time.
METHODS:
Here we present an analysis of OS for high risk pts enrolled in the CLL8, CLL10 and CLLM1 trials of the German CLL Study Group (GCLLSG). High risk was defined as either MRD positivity (≥ 10-2) in the peripheral blood after at least partial response to first-line treatment with chemoimmunotherapy or intermediate MRD level (<10-2 and ≥ 10-4) combined with either TP53 alterations or unmutated IGHV gene status. High risk pts meeting the criteria as defined above with previously untreated CLL and without relevant comorbidities enrolled in the FCR-Arm of CLL8 (between 2003 and 2006), CLL10 (between 2008 and 2011) and CLLM1 (between 2012 and 2016) studies of the GCLLSG were eligible for this analysis. Pts enrolled in CLL8 and CLL10 received up to 6 cycles of chemoimmunotherapy with fludarabine, cyclophosphamide plus rituximab (FCR) or bendamustine plus R (BR). Pts enrolled in CLLM1 were randomised (2:1) to receive lenalidomide or placebo after first-line therapy consisting of at least four cycles of FCR or BR. Details on subsequent therapies will be presented at the conference. Overall survival was defined as the time from the start date of first-line therapy to the date of death; censoring was at longest follow-up. Estimation for overall survival was done with Cox regression method and Kaplan-Meier survival curves.
FINDINGS:
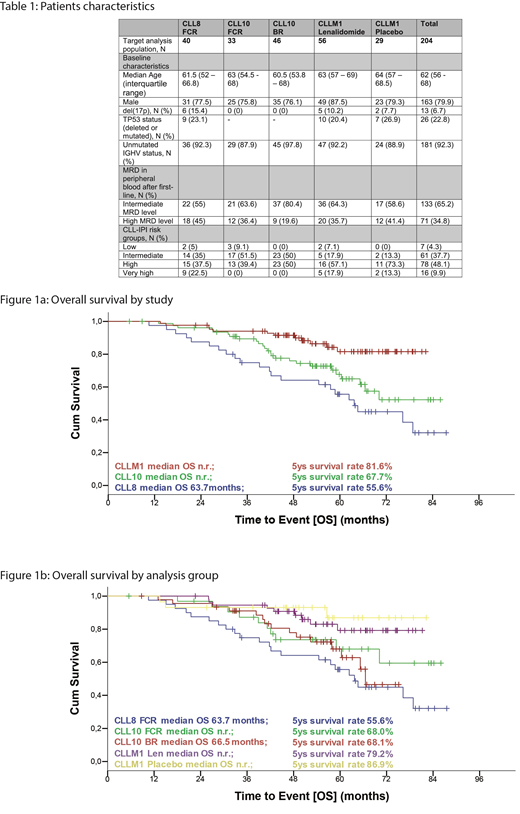
A total of 204 (CLL8, n=40 pts; CLL10, n=79 pts; CLLM1, n=85 pts) high risk pts were included in this analysis. Median observation time for the entire cohort was 59.9 months (interquartile range: 50.6-71.7). Overall, 71% of the pts are still alive, 45% of the pts in CLL8; 68% of the pts in CLL10 and 86% of the pts in CLLM1. Patient characteristics are shown in table 1. Median age was 62 years. 80% of the pts were male. Deletion of 17p was an exclusion criterion for CLL10 and was present in 15.4% of CLL8 and 9.3 % of the CLLM1 pts. TP53 mutation was not analysed for CLL10, and found in 23% (CLL8 and CLLM1) of the pts. Overall, 92.3% of the pts had an unmutated IGHV gene status. 58% of the pts had a high or very high CLL-IPI (Table 1). As per inclusion criterion for this analysis, all pts were MRD-positive, with 34.8% of a high MRD level and 65.2% of an intermediate level. Overall survival was shorter for CLL8 with a median of 63.7 months as compared to not reached in CLLM1 (HR 4.107, 95% CI: 1.983-8.141). Median overall survival for CLL10 was not reached, but shorter than the OS in CLLM1 as well (HR, 2.492, 95% CI: 1.251-4.963). Five-year survival rates were between 56% (CLL8), 68% (CLL10) and 82% (CLLM1) respectively. (Figure 1a). As compared to FCR pts in CLL8, pts in CLLM1 assigned to the lenalidomide (HR: 0.281, 95% CI: 0.129-0.612) and the placebo group (HR: 0.186, 95% CI: 0.056-0.623) had an OS advantage. As compared to CLL10 BR the OS advantage was shown for pts assigned to the lenalidomide group (HR: 0.414, 95% CI: 0.181-0.947) and the placebo group (HR: 0.274, 95% CI: 0.079-0.947) as well. (Figure 1b)
INTERPRETATION:
This analysis demonstrated an improvement for overall survival in patients with high risk CLL included in the CLLM1 study as compared to pts in the CLL8 and CLL10 trials of the GCLLSG. So far, the results suggest that progress in maintenance therapy or subsequent use of novel agents (BTK or PI3K inhibitors) may improve the outcome of high-risk CLL patients initially treated with chemoimmunotherapy.
Fink:Roche: Other: travel grants; Janssen: Membership on an entity's Board of Directors or advisory committees; Celgene: Research Funding. Kutsch:Gilead Sciences, Inc.: Research Funding; Mundipharma, AbbVie, Janssen: Other: Travel, accomodation, expenses. Wendtner:GILEAD Science: Consultancy, Honoraria, Research Funding; Janssen-CILAG: Consultancy, Honoraria, Research Funding; MorphoSys: Consultancy, Honoraria, Research Funding; Mundipharma: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding. Kreuzer:Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Mundipharma: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Tausch:AbbVie: Consultancy, Honoraria, Other: travel support, Speakers Bureau; Roche: Consultancy, Honoraria, Speakers Bureau. Stilgenbauer:Gilead: Consultancy, Honoraria, Research Funding, Speakers Bureau; GSK: Consultancy, Honoraria, Research Funding, Speakers Bureau; AstraZeneca: Consultancy, Honoraria, Research Funding, Speakers Bureau; AbbVie: Consultancy, Honoraria, Research Funding, Speakers Bureau; Celgene: Consultancy, Honoraria, Research Funding, Speakers Bureau; Novartis: Consultancy, Honoraria, Research Funding, Speakers Bureau; Pharmacyclics: Other: Travel support; Amgen: Consultancy, Honoraria, Research Funding, Speakers Bureau; Janssen: Consultancy, Honoraria, Research Funding, Speakers Bureau; Hoffmann La-Roche: Consultancy, Honoraria, Research Funding, Speakers Bureau. Ritgen:AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Consultancy, Other: travel grants, Research Funding. Böttcher:Roche: Honoraria, Research Funding; Janssen-CILAG: Honoraria, Other: Travel grants, Research Funding; Celgene: Research Funding; AbbVie: Honoraria, Other: Travel grants, Research Funding; Genentech: Research Funding; Becton Dickinson: Research Funding; Novartis: Research Funding. Fischer:Roche: Other: travel grants; AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees. Hallek:AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; GILEAD Science: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel grants, Research Funding, Speakers Bureau; Roche/Genentech: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel grants, Research Funding, Speakers Bureau. Eichhorst:Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Gilead Sciences, Inc.: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; BeiGene: Research Funding; ArQule: Membership on an entity's Board of Directors or advisory committees.
Lenalidomide is not indicated as a maintenance therapy after immunochemotherapy in patients with CLL.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal