Background
Multiple myeloma (MM), the second most common hematologic malignancy, is characterized by the expansion of malignant plasma cells which express, the cell surface protein, B-cell maturation antigen (BCMA). Patients (pts) with advanced MM who are refractory to an immunomodulatory agent (IMiD), a proteasome inhibitor (PI), and an anti-CD38 monoclonal antibody (mAb) have an expected overall survival of <1 year (Gandhi et al. 2019). Given the therapeutic potential of utilizing BCMA to redirect T-cell effector function on multiple myeloma cells, we generated REGN5458, an anti-BCMA x anti-CD3 bispecific antibody that binds to both BCMA on plasma cells and to CD3 on T-cells. Here we describe the safety and clinical activity in relapsed/refractory MM patients treated on the initial dose level of REGN5458 in trial (NCT03761108).
Methods
The primary objectives of the Phase 1 portion of the study are to determine the safety, tolerability and occurrence of dose limiting toxicities (DLTs) of REGN5458. The primary objective of the Phase 2 portion is to assess the preliminary anti-tumor activity of REGN5458. Key secondary objectives include assessment of pharmacokinetics (PK) and pharmacodynamics. Eligible pts with MM must have >3 prior lines of therapy including a PI, IMiD and anti-CD38 antibody or progression on or after an anti-CD38 antibody and refractory to a PI and IMiD. Treatment consists of 16 weekly doses of REGN5458, followed by a maintenance phase of 12 doses administered every 2 weeks. Pts with progressive disease after initial response are eligible for retreatment. Response was assessed per the International Myeloma Working Group (IMWG) criteria.
Results
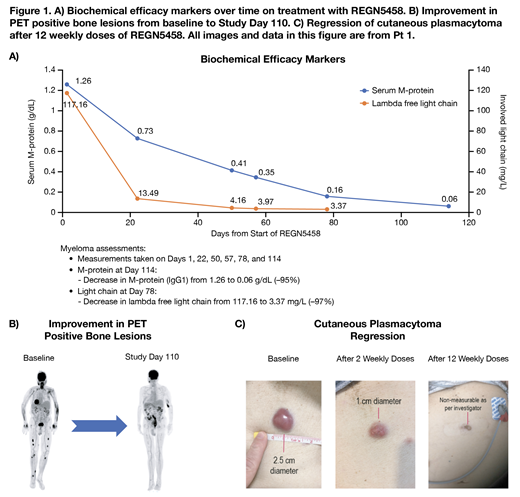
As of July 12, 2019, three pts have been treated at the initial dose level of 3 mg REGN5458. All pts had an ECOG score of 1. Pt 1, an 81-year-old male who had medullary plasmacytomas and cutaneous extramedullary plasmacytomas (EMPs), had received four prior lines of therapy. He experienced Grade (Gr) 1 cytokine release syndrome (CRS) that was treated with tocilizumab and corticosteroids because of persistent debilitating fever. This patient also experienced Gr 3 TEAEs including anemia, pain in both extremities (location of multiple sites of disease), and worsening hypertension within the DLT evaluation period. Subsequent to the DLT evaluation period, he had Gr 3 fatigue, Gr 3 febrile neutropenia, Gr 3 lung infection, Gr 3 atrial fibrillation, and Gr 4 septic shock. Pt 1 reached a partial response at Week 8 and a very good partial response (VGPR) as of Week 16 despite interruption of study drug at Week 14 due to TEAEs. This pt has IgG lambda myeloma and showed rapid decreases in both lambda free light chain and M-protein (Figure 1A) and resolution of medullary and cutaneous plasmacytomas following the Week 12 dose (Figure 1B and 1C). Pt 1 had transient cytokine elevations of interferon gamma, interleukin (IL)-6, and IL-10 following dosing through Week 5, consistent with mild CRS. Peripheral blood immune monitoring revealed increases in CD8 effector memory T-cells through Week 11, relative to other subsets which remained unchanged during the treatment period. Pt 1 remains in the treatment phase of the study.
Pt 2 is a 76-year-old female who had received four prior lines of therapy and had extensive intra-abdominal EMPs. She had no ≥ Gr 2 TEAEs. Pt 2 had disease progression at first assessment and is in the follow-up phase of the study.
Pt 3 is a 78-year-old female with seven prior lines of therapy. She experienced Gr 2 decreases in both platelets and neutrophils within the DLT evaluation period. Pt 3 had stable disease (SD) at first assessment and remains in the treatment phase of the study.
No DLTs were reported. No pt experienced infusion-related reactions. No pt had Gr 5 TEAEs or discontinued treatment due to AEs. Additional PK and biomarker data will be presented.
Conclusions/Summary
In this FIH study of REGN5458, no DLTs were recorded in the first three pts treated with the initial dose. One pt responded with a VGPR and another had SD. The study is ongoing and recruiting pts at higher doses.
Gandhi UH et al. Outcomes of patients with multiple myeloma refractory to CD38-targeted monoclonal antibody therapy. Leukemia; 2019; DOI:10.1038/s41375-019-0435-7
Madduri:Celgene: Consultancy; AbbVie: Consultancy; Foundation Medicine: Consultancy; Takeda: Consultancy. Lentzsch:Bayer: Consultancy; Columbia University: Patents & Royalties: 11-1F4mAb as Anti-Amyloid Strategy; Janssen: Consultancy; Caelum Biosciences: Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Karyopharm: Research Funding; Crossfires in hematologic Malignancies: Honoraria; International Myeloma Foundation: Honoraria; Multiple Myelopma Research Foundation: Honoraria; Abbvie: Consultancy; BMS: Consultancy; Proclara: Consultancy; Clinical Care Options: Speakers Bureau; Sanofi: Consultancy, Research Funding; Takeda: Consultancy. Jagannath:Celgene Corporation: Consultancy; Bristol-Myers Squibb: Consultancy; Merck & Co.: Consultancy; Janssen Pharmaceuticals: Consultancy; AbbVie: Consultancy; Karyopharm Therapeutics: Consultancy. Li:Regeneron Pharmaceuticals, Inc.: Employment, Equity Ownership. Boyapati:Regeneron Pharmaceuticals, Inc.: Employment, Equity Ownership. Adriaens:Regeneron Pharmaceuticals, Inc.: Employment, Equity Ownership. Chokshi:Regeneron Pharmaceuticals, Inc.: Employment, Equity Ownership. Zhu:Regeneron Pharmaceuticals, Inc.: Employment, Equity Ownership. Lowy:Regeneron Pharmaceuticals, Inc.: Employment, Equity Ownership. Weinreich:Regeneron Pharmaceuticals, Inc.: Employment, Equity Ownership. Yancopoulos:Regeneron Pharmaceuticals, Inc.: Employment, Equity Ownership. Sharma:Regeneron Pharmaceuticals, Inc.: Employment, Equity Ownership. Karasarides:Regeneron Pharmaceuticals, Inc.: Employment, Equity Ownership. Sternberg:Regeneron Pharmaceuticals, Inc.: Employment, Equity Ownership.
The data described in the abstract will report on use of REGN5458 in a first-in-human trial in patients with multiple myeloma.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal