Introduction
The incidence of recurrent venous thromboembolism (VTE), encompassing deep vein thrombosis (DVT) and pulmonary embolism (PE), is dependent on multiple patient demographic factors and medical co-morbidities, such as cancer and surgery. We sought to determine risk factors associated with 6-month cumulative incidence of recurrent VTE.
Methods
A detailed description of the population-based surveillance systems have been previously published (Wendelboe 2015 and Ortel 2019 pending publication). In brief, the Centers for Disease Control and Prevention collaborated with University of Oklahoma Health Science Center and Duke University to establish surveillance systems that utilized active and passive methods to obtain data on all VTE events that occurred within Oklahoma and Durham counties, respectively between April 2012 and March 2014. This is the first report combining data from the two surveillance systems.
Eligibility for the current analysis included 1) patients 18 years or older at the time of index VTE 2) no reports of patient death between index VTE and recurrent VTE or within 6 months of index VTE 3) patient data from a hospital system or out-patient clinics associated with a hospital (i.e. individuals treated at non-hospital-based out-patient clinics were excluded due to missing treatment data) 4) and index VTE occurred at least 6 months prior to the end of the surveillance period or a 6-month follow-up data abstraction was performed by the site.
Recurrent VTE was defined as 1) VTE (either DVT or PE) in a different location and diagnosed after the index VTE or 2) VTE in the same location and diagnosed greater than 90 days after index VTE.
Results
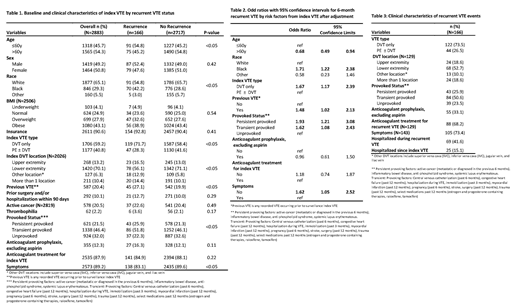
During the surveillance period 4,205 patients were diagnosed with an index VTE, of these 2,883 (68.6%) were eligible for analysis. The 6-month incidence of VTE recurrence was 5.8% (n=166). Recurrent VTE events were diagnosed within 3 to 179 days post index VTE. Compared to patients without recurrent VTE, patients with recurrent VTE were more likely to be younger (≤ 60 years of age) and black (Table 1). They were also more likely to have a DVT only as their index VTE, to have a provoked VTE, and to have had a VTE prior to their index VTE; they were less likely to present with symptoms at the time their index VTE was diagnosed. The majority of index DVTs were located in the veins of the lower extremities, however among patients with recurrent DVT there was an increased proportion of index DVTs diagnosed in the upper extremities, other locations, and in more than one location. These differences remained after multivariable adjustment (Table 2).
Clinical characteristics of recurrent VTE events are summarized in Table 3. Recurrent VTEs were primarily DVT only (73.5%), located in the lower extremity (52.7%), symptomatic at presentation (73.4%), and were associated with transient provoking factors (50.6%) including hospitalization (41.6%). Several patients (n=25) were hospitalized from the date of their index VTE to the date of VTE recurrence. Use of pharmacologic prophylaxis was high at time of recurrence (33.1%) compared to use at index VTE (12.3%).
Discussion
Currently, there is no U.S. national VTE surveillance system. Our VTE surveillance results show significant differences in the risk of VTE recurrence according to both patient demographic factors and clinical features of the index VTE. Factors associated with higher recurrence risk include 60 years or younger, black race, and index VTE that was DVT only, asymptomatic, and associated with persistent or transient provoking factors. Fatal recurrent VTEs may not have been identified at time of death, potentially underestimating the VTE recurrence incidence and influence of risk factors. The proportions of recurrent VTE events reported here is similar to the proportions reported in previous cohort studies, indicating that these population-based surveillance systems captured most recurrent VTE events among patients seen within hospital systems for their index VTE.
Ortel:Instrumentation Laboratories: Consultancy. Raskob:Bayer Healthcare: Consultancy, Honoraria; Daiichi Sankyo: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Tetherex: Consultancy; Novartis: Consultancy; Anthos: Consultancy; Janssen R&D, LLC: Consultancy, Honoraria; Boehringer Ingelheim: Consultancy; BMS: Consultancy, Honoraria; Portola: Consultancy; Eli Lilly: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal