Introduction: Multiple Myeloma (MM) predominantly affects older patients, and treatment in older patients is fraught with challenges due to the spectrum of aging-associated vulnerabilities present in older patients. Age-associated cumulative decline across physiological systems results in a diminished resistance to stressors, including cancer and its treatment, creating a vulnerable state known as frailty. Frailty is associated with increased risk of adverse outcomes in patients with cancer. Identification of frailty in retrospective data, including the electronic medical record, can allow for assessment of prognosis, provide input during treatment selection and facilitate control for confounding, as in comparative effectiveness research. A frailty index (FI) operationalizes frailty using an accumulation of health-related deficits (Rockwood 2007). The deficits accumulation approach to frailty is validated in many populations and settings, including in older patients with cancer receiving chemotherapy and older adults with myeloma. Routinely collected health information in the electronic medical record system may provide a standardized and feasible means of identifying cumulative deficits and generating a FI in older adults with myeloma. The objective of this study was to develop a claims-based FI in veterans with MM.
Methods: From the Veterans Administration Central Cancer Registry (VACCR), we identified all patients aged 65 years and older diagnosed with MM between 1999 and 2014. For the construction of the FI using an accumulation of health-related deficits model, we followed the method described by Orkaby et al (Orkaby, J Gerontol A Biol Sci Med Sci, 2018). The FI is calculated by dividing the total number of deficits an individual has by the total number of possible variables, giving a score between 0 and 1. We included 31 deficits in a range of systems including comorbidity, functional status, cognition, mood, sensory loss, and other geriatric domains. We categorized the FI into five groups as recommended: non-frail (FI 0-0.1), pre-frail (0.11-0.20), mild frailty (0.21-0.30), moderate frailty (0.31-0.40), and severe frailty (>0.4). We used Kaplan-Meier curves to demonstrate the relationship between frailty status and mortality in patients with MM. Next, we used cox proportional hazards regression to control for the following factors in the mortality analyses: age, BMI, race, diagnosis year, MM treatment, bisphosphonate use, and statin use.
Results: We identified 3807 patients aged 65 years and older diagnosed with MM.
Prior to starting treatment for MM, 28.7% of the cohort was classified as non-frail, 41.3% as pre-frail, 21.6% as mildly frail, 6.6% as moderately frail, and 1.7% as severely frail.
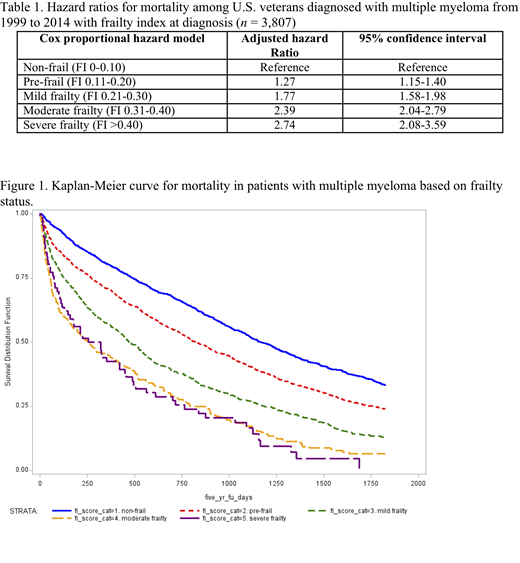
Increasing frailty status was associated with increased mortality (Figure 1). The median overall survival for non-frail veterans was 38 months, compared to those who were pre-frail (27 months), mildly frail (15 months), moderately frail (8 months), and severely frail (9 months). After controlling for the relevant variables, higher frailty score remained significantly associated with higher mortality (Table 1).
Conclusions: In this study, we demonstrate a systematic method of calculating a FI using the readily available administrative and claims data from the Electronic Medical Record in veterans with MM. Frailty status was strongly associated with mortality, independent of age, race, BMI, MM treatment, and statin use. Assessment of frailty status using the readily available electronic medical records data in retrospective data allows for assessment of prognosis.
Sanfilippo:Bristol-Myers Squibb: Speakers Bureau; Pfizer: Membership on an entity's Board of Directors or advisory committees; Bayer: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Other: Travel Expenses; NHLBI: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal