BACKGROUND AND AIM
Bone marrow compensation in autoimmune hemolytic anemia (AIHA) is an emerging predictor of clinical outcome. It is measured by reticulocytosis that may be inadequate in a proportion of cases, particularly in chronic refractory ones. Moreover, reticulocytosis may be masked by constant destruction, particularly in cold forms. Recombinant erythropoietin (EPO), has been anecdotally used off-label in AIHA to improve anemia, but only case reports and small series have been described. Here we evaluate EPO efficacy and predictors of response in a multicentric cohort of primary and secondary AIHA patients.
METHODS
Hematological data, hemolytic markers (LDH, reticulocytes), and concomitant treatments were retrospectively and prospectively collected. Efficacy was evaluated at 15 and 30 days, and then at 3,6 and 12 months after EPO start. Response was considered as partial (PR, >2 g/dL Hb increase or >10 g/dL) or complete (CR, >12g/dL and normalization of hemolytic markers). Forty-six AIHA cases followed from June 2007 to June 2019 at 9 centers in Italy, France, Norway, Austria, Denmark, and UK were included.
RESULTS
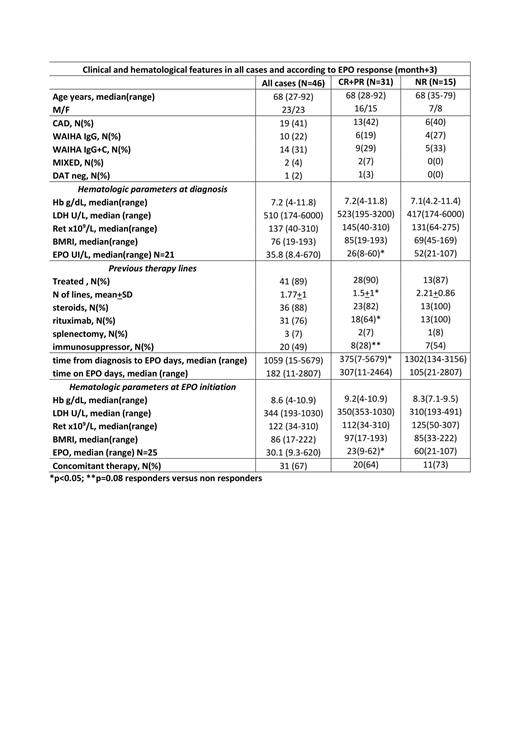
Table 1 shows patients characteristics: all AIHA types (warm, cold, mixed, and DAT negative) were present, and 5 cases were secondary to a lymphoproliferative disorder (not active and without specific treatment at the time of EPO start). Bone marrow evaluation pre-EPO (N=24) showed hypercellularity in 14 cases, dyserythropoiesis in 11, and reticulin fibrosis in 3; a lymphoid infiltrate was found in 19 patients (T-cell in 6, B-cell in 10, mixed in 3), greater than 10% in the 5 secondary cases only. Forty-one cases (89%) had been previously treated, with a mean of 1.8+1 lines of therapy including steroids, rituximab, splenectomy, immunosuppressors and sutimlimab (1 case, where the drug completely abolished hemolysis). The majority (67%) started EPO due to non-response to ongoing treatment (18 steroids, 6 immunosuppressors, 1 sutimlimab) or within 3 months from rituximab course (7). At EPO initiation, 30% of cases displayed severe anemia, 71% had inadequate reticulocytosis (bone marrow responsiveness index<121), and 73% showed inappropriately low endogenous EPO levels. Of note, 2 patients had concomitant renal impairment, possibly contributing to this finding. Most cases received epoetin alpha 40,000 UI/week (45%), followed by darbopoetin alpha (34% of cases, median dose 102 mcg/week) and epoetin zeta (14% of cases, 30,000 UI/week). EPO was administered for a median of 6 months and responses were observed in 68% and 70% of cases at month+1 and +3. Comparable response rates were recorded at month+6 (70%, 13 CR and 1 PR, N=20) and +12 (72%, 8 CR and 5 PR, N=18), although evaluable cases were fewer. Median Hb increase from baseline was 2.5 g/dL (0.2-7.6) at month+1 (p<0.001), and 3.1 g/dL (0-9.4) at month+3 (p<0.001). Consistently, reticulocytes increased by 23 x109/L (0-217) at month+1, and 33 x109/L (0-353) at month+3. No EPO-related adverse events occurred (particularly no thrombosis). At last follow up, 23 cases had discontinued EPO: 13 for long lasting CR and 10 because of NR. Considering predictors of response, a better efficacy was observed in primary versus secondary AIHA (71 vs 40%) and in patients with shorter time from diagnosis to EPO treatment (52% of responders started EPO within 1 year from diagnosis vs 8% of NR, p=0.01). Moreover, responders had received a lower number of previous treatments (p=0.04), particularly rituximab (p=0.05) and immunosuppressors (p=0.08). Remarkably, responders more frequently showed severe anemia (86% vs 62%) and lower endogenous EPO (91% vs 50% with a cut-off of <60 UI/L, p=0.05) at baseline.
CONCLUSIONS
EPO is effective in roughly 70% of chronic refractory AIHA cases, independently from antibody thermal characteristics/isotype and underlying disease. Concomitant treatments may partially affect response evaluation, although EPO treatment has been introduced because of their partial or complete inefficacy. Further limitations are the retrospective nature of the study and a possible selection bias (i.e. most of patients had inadequate reticulocytosis). Predictors of response were severe anemia and low levels of endogenous EPO, as well as, shorter disease duration and a lower burden of previous treatments. These data suggest an early use of EPO in this setting in order to overcome inadequate bone marrow compensatory ability.
Fattizzo:Apellis: Consultancy. Michel:Novartis: Consultancy; Amgen: Consultancy; Rigel: Consultancy. Frederiksen:Novartis: Research Funding; Janssen Pharmaceuticals: Research Funding; Abbvie: Research Funding; Alexion: Research Funding; Gilead: Research Funding. Mauro:Gilead: Consultancy, Research Funding; Shire: Consultancy, Research Funding; Abbvie: Consultancy, Research Funding; Jannsen: Consultancy, Research Funding; Roche: Consultancy, Research Funding. Jilma:TrueNorth a Bioverativ company, a Sanofi company: Consultancy, Research Funding. Hill:Regeneron: Honoraria; Roche: Honoraria; Ra Pharma: Honoraria; Bioverativ: Honoraria; Novartis: Honoraria; Akari: Honoraria; Alexion: Honoraria; Apellis: Honoraria. Berentsen:Mundipharma: Research Funding; Apellis, Bioverativ (a Sanofi company), Momenta Pharmaceuticals, and True North Therapeutics: Consultancy; Alexion, Apellis, and Janssen-Cilag: Honoraria. Barcellini:Agios: Other: Advisory board; Bioverativ: Other: Advisory board; Alexion: Other: Invited Speaker, Research Funding; Novartis: Other: Invited Speaker, Research Funding; Incyte: Other: Advisory board.
Erythropoietin (EPO) is not yet indicated for the use in autoimmune hemolytic anemia. Here we report retrospective data on a large cohort of cases treated with EPO as a support to bone marrow compensation.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal