
Introduction
Kidney iron deposition has been described in hemolytic disorders including mechanical valves, paroxysmal nocturnal hematuria, and sickle cell disease (Roberts & Morrow, Circulation 1966; Leonardi & Ruol, Blood 1960). Circulating plasma hemoglobin is filtered at the glomerulus and reabsorbed via the megalin cubulin system in the proximal and distal tubules (Gburek et al, J Am Soc Nephrol 2002). On MRI, this manifests as signal loss on gradient and spin echo sequences in the cortex of the kidney with complete sparing of the medulla (Jeong et al, Radiographics 2002). The signal darkening is quantified by the parameter R2*, which has been shown to be directly proportional to tissue iron in the liver and heart. Kidney R2* has previously been demonstrated to rise proportionally to lactate dehydrogenase (LDH) in chronically transfused sickle cell disease (SCD) patients (Wood et al, Br J Haematol 2016; Schein et al, J Magn Reson Imaging 2008), but LDH is not a specific marker of hemolysis, and chronically transfused patients could potentially deposit iron in the kidney through other mechanisms. Therefore, we characterized the relationship between kidney R2*, urinary iron and markers of hemolysis in non-transfused SCD patients.
Methods
Sixty-five non-transfused SCD patients were recruited to the study, which was approved by the Institutional Review Board of Children's Hospital Los Angeles. Following medical history and physical exam, subjects completed blood and urine testing, and then abdominal MRI for assessment of somatic iron stores. R2* measurements were collected using multiple gradient echo pulse sequences on 1.5 Tesla magnets. Statistical analysis was performed using JMP® Pro, Version 14.0.0 (SAS Institute Inc., Cary, NC, 2018).
Results
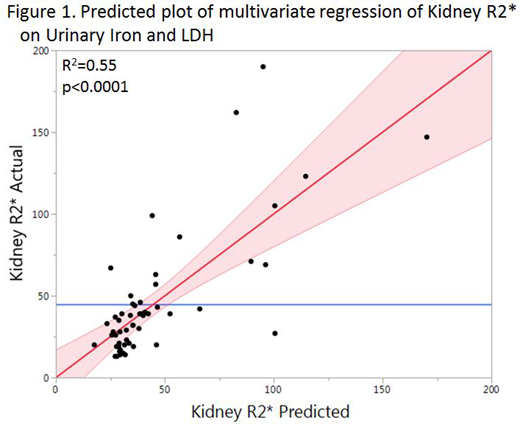
Subjects were generally adults with a mean age of 32 years. Nearly three quarters of subjects had homozygous sickle cell disease, while a quarter had SC disease or S-Beta thalassemia, and one subject had sickle cell trait. Most subjects were anemic, and all subjects had elevated markers of hemolysis. Fifty-four percent of subjects had an elevated kidney R2* level (≥34 Hz). On univariate analysis, kidney R2* was associated with urinary iron (R2=0.52, p<0.0001), LDH (R2=0.33, p<0.0001), plasma hemoglobin (R2=0.18, p=0.0007), and hemoglobin (R2=0.08, p=0.02), but it was not associated with reticulocyte count. On multivariate analysis, kidney R2* was associated with urinary iron and LDH (Figure 1). No association was found between R2* values of the kidney, liver, and pancreas.
Discussion
Our study supports previous findings that kidney R2* is associated with intravascular hemolysis, as measured by plasma hemoglobin and LDH, and inversely by hemoglobin (Kato et al, Blood 2006). The stronger association with urinary iron reinforces the concept that kidney R2* reflects filtered iron, which is insufficiently reabsorbed due to proximal and distal tubular injury in iron overload (van Raaij et al, Am J Physiol Renal Physiol 2019). Urinary iron is also elevated in diabetic nephropathy (Van et al, J Am Soc Nephrol 2017) and nephrotic syndrome (Niel et al, Blood 2011). These data raise important questions regarding the possible role of iron in sickle related renal disease.
Coates:apo pharma: Consultancy, Honoraria, Speakers Bureau; vifor: Consultancy, Honoraria; agios pharma: Consultancy, Honoraria; celgene: Consultancy, Honoraria, Other: steering committee of clinical study. Wood:National Institutes of Health: Research Funding; Philips Healthcare: Research Funding; BluebirdBio: Consultancy; Celgene: Consultancy; Apopharma: Consultancy; WorldcareClinical: Consultancy; BiomedInformatics: Consultancy; Imago Biosciences: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal