Introduction: Defibrotide is a polydisperse mixture of predominantly single-stranded polydeoxyribonucleotide sodium salts derived from porcine intestinal mucosa. It is currently the only therapy FDA-approved to treat hematopoietic stem cell transplant (HSCT)-associated hepatic veno-occlusive disease (VOD) with multi-organ dysfunction. Its mechanisms of action are complex and incompletely understood. In vitro, defibrotide can: promote endothelial cell (EC) mitogenesis via interaction with basic fibroblast growth factor; inhibit platelet adhesion and aggregation; reduce calcineurin inhibitor-induced EC apoptosis; and block pro-inflammatory cytokine activity, in the absence of anticoagulant effects (Blood Adv 2018;2:1495-1509). Given the critical role of EC injury with impaired regulation of thrombo-fibrinolytic pathways in both transplant-associated VOD and thrombotic microangiopathies (TMA), defibrotide has been used off-label to treat TMAs post-allogeneic HSCT. Significant clinical responses occurred in the majority of cases not complicated by renal failure (Bone Marrow Transplant 2019;54:142-145). It is known that conditioning regimens for autologous HSCT involve activation of pro-inflammatory pathways in EC which can be blocked by defibrotide (Biol Blood Marrow Transplant 2011;17:497-506). We sought to determine whether clinically relevant concentrations of defibrotide could block activation of caspase 8 in EC, an early step in apoptotic injury, as induced by plasmas from patients with TMAs secondary to thrombotic thrombocytopenic purpura (TTP), cancer/chemotherapy, and allogeneic HSCT.
Methods: We utilized plasmas from individuals with acute TMAs: 13 with TTP; 5 with an atypical hemolytic-uremic syndrome (HUS)-type of TMA in the setting of cancer chemotherapy, persisting after withdrawal of chemotherapy; and 10 with TMAs following alloHSCT, persisting after withdrawal of GvHD prophylaxis. The in vitro model system was previously published by our lab, documenting the ability of plasmas from acute TTP patients to activate caspase 8 in EC, followed by apoptotic EC death (Blood 2008;112:340-349). This effect was dependent on plasma-based proinflammatory cytokines capable of down-regulating EC cytoprotective factors. Briefly, 5 x 105 primary human neonatal microvascular endothelial cells of dermal origin were plated in 1ml culture medium/well of 12 well polystyrene plates (3.6cm2/well) pre-coated with 1% fibronectin. 2% (v/v) patient or control plasma was added, alone or with varying concentrations of defibrotide (0.5-10μg/ml), for 3hrs at 37ºC. Cells were then harvested and a commercial colorimetric kit (Millipore) was used to assess caspase 8 enzyme activity.
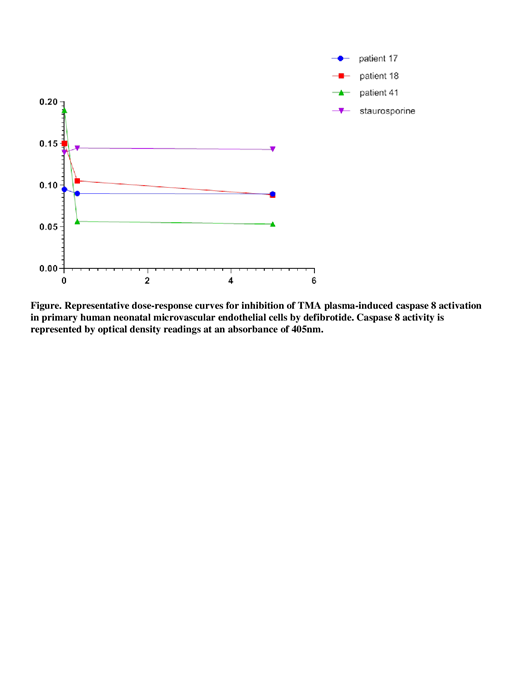
Results: Initial dose-response curves showed a plateau effect for defibrotide-mediated inhibition of caspase 8 activation at 5μg/ml; subsequent experiments utilized that dose. To control for the possibility of direct inhibition of caspase 8 by defibrotide, we found that caspase 8 activity initiated by staurosporine (1μM) was unaffected by defibrotide (Fig.) >50% inhibition of caspase 8 was seen for 3/5 cancer/chemotherapy-linked TMA patient plasmas, 7/13 TTP plasmas, and 0/10 TA-TMA plasmas. >20% suppression of such activation was seen for 5/5 cancer/chemotherapy TMA plasmas, 12/13 TTP plasmas, and 4/10 TA-TMA plasmas. Representative examples are shown in the Fig., including defibrotide blocking caspase 8 activity induced by plasmas from a patient with cancer/chemotherapy linked TMA (patient 18) or TTP (patient 41), but not from another TTP patient (patient 17).
Conclusions: Interventions with high degrees of therapeutic success are available for TMAs related to TTP and primary aHUS. However, TMAs complicate 20% of alloHSCT, and though half may resolve when GvHD immunoprophylaxis is discontinued, three year survival rates for those in whom the TMA persists are as low as 11%. Based on multiple case reports, defibrotide may represent an important resource in those cases, and in other TMAs refractory to conventional treatment. Further studies are required to see if defibrotide-mediated blockade of plasma-induced EC activation and injury which we demonstrate in vitro correlates with in vivo responses, and if the mechanism involves suppression of pro-inflammatory cytokine-mediated cell activation.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal