DuoBody-CD3xCD20 (GEN3013) is a novel clinical-stage CD3 bispecific antibody (bsAb) targeting CD20-positive tumor cells. GEN3013 was previously shown to induce potent T cell-mediated cytotoxicity towards B cell Non-Hodgkin lymphoma (B-NHL) cell lines in vitro and in vivo. Here, we investigated the cytotoxic activity of GEN3013 in tumor cells obtained from lymph node (LN) biopsies of B-NHL patients, who were newly diagnosed (ND) or relapsed from/refractory to (RR) treatment regimens containing CD20 monoclonal antibodies. Moreover, we explored whether specific tumor microenvironment characteristics could be associated with sensitivity to GEN3013.
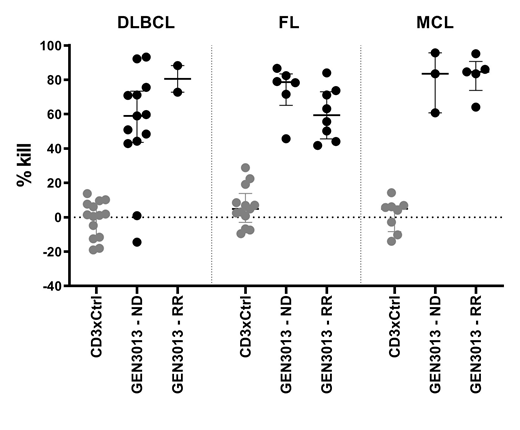
To test the intrinsic susceptibility of B-NHL cells to GEN3013, independent of interpatient variation in tumor T cell frequency or activation status, single cell suspensions obtained from LN of B-NHL patients were incubated with GEN3013 in the presence of allogeneic PBMC from a single donor, at an effector to target (E:T) ratio 10:1. GEN3013 (30 ng/mL) induced median tumor cell lysis of 64% in Diffuse Large B Cell Lymphoma (DLBCL, n=14), 69% in Follicular Lymphoma (FL, n=14) and 84% in Mantle Cell Lymphoma (MCL, n=8) samples, with EC50 values ranging from 0.01-3.9 ng/ml. Importantly, cytotoxic activity of GEN3013 was comparable in ND (n=24) and RR (n=12) patients (Figure 1). In these assays considerable heterogeneity in T cell activation, as assessed by expression of CD25, CD69 and granzyme B release, was observed. Furthermore, high expression of T cell activation markers was not always associated with high levels of GEN3013 cytotoxic activity, suggesting tumor-intrinsic resistance mechanisms.
In parallel, in all B-NHL samples GEN3013-mediated cytotoxicity was assessed without the addition of allogeneic PBMCs, thus purely relying on T cells present in the LN biopsy. In this setting, median tumor cell lysis was lower; 18% in DLBCL (range 0-46%), 17% in FL (range 0-46%) and 0% in MCL (range 0-11%), but strongly correlated with the number of T cells present in the single cell suspensions. Analysis of the tumor microenvironment by 7 color immunohistopathology of matched FFPE-embedded tumor biopsies (n=24), confirmed that the T cell frequency in the tumor biopsies was the major determinant of GEN3013 cytotoxic activity in DLBCL, FL and MCL. Moreover, experiments using (MACS) purified T cells from 4 DLBCL and 5 FL LN biopsies demonstrated that the intrinsic capacity of tumor LN T cells to induce GEN3013 mediated cytotoxicity was comparable to healthy donor T cells. Detailed tumor microenvironment analysis based on 7 color immunohistopathology staining, including relative frequency and spatial distribution of CD4 and CD8 T cells and macrophages, as well as the T cell activation status, in relation to sensitivity to GEN3013 mediated tumor cell lysis is ongoing and results will be presented.
In conclusion, GEN3013 induced potent cytotoxicity in tumor cells of DLBCL, FL and MCL patients ex vivo, irrespective of prior treatment with CD20 monoclonal antibodies. Autologous T-cells at the tumor site were able to mediate GEN3013-induced cytotoxicity, and cytotoxic activity was enhanced in presence of PBMCs suggesting that optimal tumor cell kill by GEN3013 is dependent on T-cells in the tumor microenvironment. The cytotoxic capacity of B-NHL patient T cells within the tumor microenvironment was comparable to healthy donor peripheral blood T cells, emphasizing the therapeutic potential of CD3 bsAb in B-NHL. A First-in-Human trial to assess the safety and preliminary efficacy of GEN3013 in B-NHL patients is currently ongoing (NCT03625037).
Figure1 Cytotoxic activity induced by GEN3013 compared to CD3xcontrol bsAb (both 30ng/ml) towards tumor cells obtained from lymph node (LN) biopsies of newly diagnosed (ND) versus relapse or refractory (RR) DLBCL, FL and MCL patients. GEN3013 achieved comparable lysis in ND versus RR patients (Mann-Whitney U test; not significant). Error bars represent median ± interquartile range.
Van Der Horst:Genmab: Other: Financial Support. Hiemstra:Genmab: Employment, Equity Ownership, Other: Warrants. de Jong:Genmab: Research Funding; BMS: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees. Chamuleau:Genmab: Research Funding. Zweegman:Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Research Funding. Breij:Genmab: Employment, Other: Warrants. Roemer:Genmab: Research Funding. Mutis:Celgene: Research Funding; Janssen Research and Development: Research Funding; Onkimmune: Research Funding; Genmab: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal