Background
Treatment-free remission (TFR) in patients with chronic myeloid leukemia (CML), who achieve a sustained and deep molecular response (DMR, MR4 or MR4.5) after treatment with BCR-ABL1 tyrosine kinase inhibitors (TKIs), is possible after TKI discontinuation (Rea and Cayuela. 2018). Nilotinib has been shown to elicit deeper and faster molecular responses compared to imatinib. More than 50% of patients who responded to imatinib, but did not achieve a DMR, achieved a DMR 1 year after they switched to nilotinib (Hughes et al. 2017). On attempting TFR, about 50% of patients lose response early and relapse (Mahon et al. 2019). The reason for this loss of response after achieving sustained DMR in patients has been attributed to residual and quiescent LSCs (Houshmand, et al. 2019). LSC for CML arises from the transformation of a hematopoietic stem cell through a t(9;22) translocation, leading to the Philadelphia chromosome [Ph1], and is characterized by the presence of the unregulated tyrosine kinase activity of the chimeric BCR-ABL1 protein together with specific surface antigens including CD26 and/or other aberrant markers (Bocchia, et al. 2018).
The ENESTPath study is designed to evaluate the impact of duration of 24 or 36 months (mos) of consolidation treatment with nilotinib after switching from imatinib, on the rate of TFR. The LSC sub-study was designed to evaluate the correlation between duration of nilotinib therapy and LSCs. This interim analysis of the LSC sub-study presents preliminary data on characteristics of LSC-positive patients and their response after 24 mos of nilotinib therapy.
Objectives
To assess factors impacting LSCs and the effect of nilotinib therapy in patients with CML who switched from imatinib to nilotinib.
Methods
Patients from the ENESTPath study who switched from imatinib received 24 mos of nilotinib therapy (12 mos of induction and consolidation therapy, each). Patients who achieved stable MR4 (4/5 preceding quarterly real-time quantitative polymerase chain reaction [RT-PCR] assessments were ≥MR4 and last assessment was ≥MR4) at the end of the consolidation phase were randomized 1:1 to receive either an additional 1 year of nilotinib treatment or to start TFR, while those without stable MR4 were not randomized (NR), and followed-up until end of the study (5 years after baseline visit). For the current analysis, bone marrow samples collected at screening and at 24 mos were evaluated for the presence of Ph+ on fluorescence-activated cell sorting-purified CD34+CD38-/+ cells by fluorescence in situ hybridization and/or RT-PCR techniques.
Results
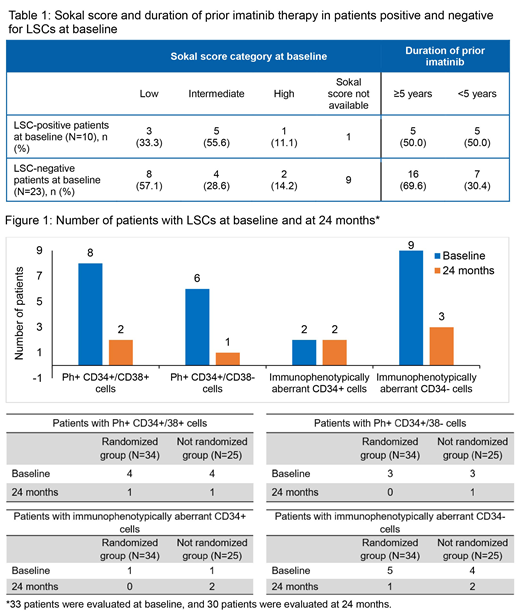
Of the 60 patients enrolled in the sub-study, 34 were randomized, 25 were NR, and 1 had protocol deviation (not included in the analysis). Overall, the mean age of the patients was 49.4 years. All patients had been previously treated with imatinib for a median duration of 59.7 mos. When analyzed for LSCs by RT-PCR or histone (HIS) at baseline, 10 patients were found to be positive, 23 were negative, 25 patients had data missing, and 1 was not evaluable. Sokal score categories and duration of prior imatinib therapy for LSC-positive and negative patients at baseline are presented in Table 1. More patients with low Sokal risk and longer duration of prior imatinib therapy (≥ 5 years) were negative for LSCs at baseline (Table 1).
After treatment with nilotinib, the number of patients with Ph+ among CD34+CD38-, CD34+CD38+, and CD34+CD38-/+ LSCs was lower at 24 mos compared to baseline, except for aberrant CD34+ cells in the NR group (Figure 1). A decrease in the percentage of CD34+/CD38+ and immunophenotypically aberrant CD34+/- cells was observed for most of the patients analyzed at baseline and at 24 mos by HIS or RT-PCR. Overall, 11 and 10 patients in the randomized and NR groups, respectively, had ≥1 dose reduction/interruptions; adverse events led to dose reduction/interruptions in 9 patients each, in both arms.
Conclusion
This analysis indicates that treatment of CML patients with nilotinib after switching from imatinib may play a role in the reduction of LSCs over time. As the number of patients with available Sokal score at baseline and prior imatinib exposure was low, meaningful conclusions cannot be drawn, and further investigations are needed. Results of patients attempting TFR are awaited, and it would be interesting to analyze the correlation between the LSC count and rate of TFR in the final analysis of the sub-study.
Sanchez-Guijo:BMS: Consultancy, Honoraria; Incyte: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Amgen: Honoraria; Roche: Honoraria. Plata:Novartis: Consultancy, Speakers Bureau; Takeda: Consultancy, Speakers Bureau; Sanofi: Consultancy, Speakers Bureau; Amgen: Consultancy, Speakers Bureau. Crugnola:Novartis: Honoraria; Incyte: Honoraria. Caocci:Novartis: Honoraria; Celgene: Honoraria. Oakervee:Novartis: Honoraria; Bristol Myers-Squibb: Honoraria; Pfizer: Honoraria. Jedrzejczak:Amgen: Consultancy; Takeda: Consultancy; Novartis: Research Funding; Amgen: Other: Travel support for hematology meetings (ASH, EBMT, EHA) ; Celgene: Other: Travel support for hematology meetings (ASH, EBMT, EHA); Roche: Other: Travel support for hematology meetings (ASH, EBMT, EHA). Kiani:Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Supekar:Novartis: Employment. Ferreira:Novartis: Employment. Shah:Novartis: Other: Service Provider. Steegmann:BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Baccarani:Novartis: Consultancy, Speakers Bureau; Incyte: Consultancy, Speakers Bureau; Takeda: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal