Background:
Mutational profiling using next-generation sequencing (NGS) has enhanced our biological understanding of and improved prognostic abilities for patients with BCR-ABL1-negative myeloproliferative neoplasms (MPN). Mutations in JAK2, CALR, and MPL, all of which activate the JAK-STAT pathway, account for the majority of MPN driver mutations, and are thought to be mutually exclusive. However, recent data has demonstrated that multiple JAK-STAT activating mutations may coexist in the same patient. Whether such patients demonstrate unique disease biology or clinical manifestations of disease has not been elucidated. Furthermore, characterization of the genomic architecture and histopathologic details of such cases has largely not been described.
Methods:
We queried genomically-annotated clinical databases at Mount Sinai Hospital and Memorial Sloan Kettering Cancer center to identify patients with myeloid malignancies with dual driver mutations in either JAK2, CALR, and MPL, with absence of BCR-ABL1. We performed chart review to record clinical parameters. Sequencing was carried out using a commercial CLIA-certified myeloid malignancy NGS panel (Genoptix, N=2) or a CLIA-certified myeloid/lymphoid malignancy NGS panel at Memorial Sloan Kettering (N=10). Only variants deemed to be oncogenic or likely oncogenic based on published reports or publicly-available annotated databases were included. We assigned cytogenetic risk for primary myelofibrosis (PMF) based on DIPSS. Histopathologic analysis was performed on bone marrow biopsy and aspirate samples simultaneous with mutational profiling.
Results:
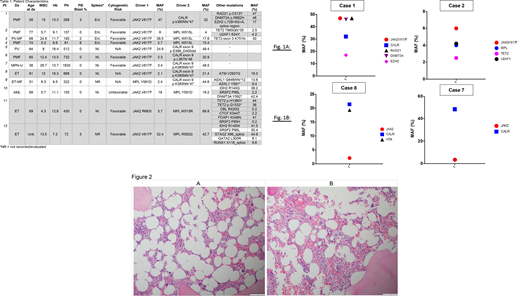
We identified 11 MPN patients and 1 de novo AML patient out of a total of 680 sequenced patients, with two JAK-STAT activating mutations (Table 1). No cases were identified with more than two activating JAK-STAT mutations. We identified canonical mutations in JAK2, MPL, and CALR in most cases, with some cases harboring mutations reported at a lower frequency in these genes. Co-occurring mutations in genes frequently identified in myeloid malignancies such as TET2 and splicing factors were also identified. Clonal architecture was inferred from mutant-allele fraction (MAF) ratios in these cases. We identified two predominant patterns of clonal architecture across these cases. First, cases in which the MAFs of the two JAK-STAT driver mutations approximate each other (Figure 1A) and second, cases in which one of the JAK-STAT mutations represents the dominant clone (Figure 1B). The greatest discordance in driver mutation MAF occurred in patients with ET, whereas several cases of PMF demonstrated a relative concordance of driver mutation MAF. Karyotype was normal in 4 patients and not performed in 3 patients; the remaining patients had: gain of 9, t(7;12), del(16q), and +der(9)(p10),del(9)(p12p21). Additionally, aCGH+SNP uncovered cryptic CNLOH of 7q22.37q31.2 in one patient. Histopathologic analysis of these cases demonstrated findings consistent with classical description of MPNs (Figures 2A, 2B). These histologic sections from a patient with concurrent MPL and JAK2 mutations showed features suggestive of ET. However, megakaryocytes showed variable morphology ranging from bulbous forms to slightly hyperlobulated forms. Overall, the megakaryocytes were not overly enlarged.
Conclusions:
Consistent with prior observations, in a proportion of cases with dual driver mutants, the MAF between the two genes is highly discordant. However, in a substantial proportion of cases we have identified, the MAFs of co-occurring driver mutations were relatively concordant. These findings suggest the possibility of clonal interference, as has been described in RAS-mutant core-binding factor AML (Itzykson R, et al. Blood 132.2 (2018): 187-196). Co-existing clonal hematopoiesis could also explain these results. These findings may have clinical implications for the natural history of the disease. Of greater importance, this may have implications for response to therapeutic modalities such as interferon, as differences in response rates to interferon have been described based on the type of JAK-STAT driver mutation present. Thus, clonal selection may occur. Clinical and preclinical data pertaining to the impact of interferon and JAK inhibitors on clonal architecture in dual JAK-STAT driver mutation cases will be presented at the meeting.
Arcila:Invivoscribe, Inc.: Consultancy, Honoraria. Mascarenhas:Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Research Funding; Roche: Consultancy, Research Funding; Merck: Research Funding; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; CTI Biopharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Research Funding; Promedior: Research Funding; Merus: Research Funding; Pharmaessentia: Consultancy, Membership on an entity's Board of Directors or advisory committees. Hoffman:Merus: Research Funding. Rampal:Celgene: Consultancy; Jazz: Consultancy; Blueprint: Consultancy; Constellation: Consultancy, Research Funding; Stemline: Consultancy, Research Funding; Incyte: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal