Background: GRP78, an endoplasmic reticulum stress-inducible molecular chaperone, is up-regulated at times of cellular stress to limit proteotoxicity and promote cell survival. Translocation of GRP78 to the cell surface (csGRP78) is emerging as a critical step providing tumour cells with a survival advantage. Here we quantified, monitored and correlated plasma cell (PC) csGRP78 expression in patients (pts) with relapsed/refractory multiple myeloma (RRMM) treated with carfilzomib, thalidomide and dexamethasone (KTd).
Method: Patients enrolled in the single arm, multicentre, phase II Australasian Leukaemia & Lymphoma Group MM018/Asian Myeloma Network 002 study were treated with KTd as described previously (Quach et al. Blood 2018 132:1955). Formalin-fixed, paraffin-embedded BM trephine sections collected at baseline (n=29), after 6-months (mo) of KTd (n=19) and at time of disease progression (PD; n=5) were stained for CD138 and GRP78 by multiplex immunofluorescence histochemistry using the OpalTM workflow. Membrane expression of CD138 and GRP78 was extracted using inForm® software, compared across timepoints and correlated to disease characteristics and treatment outcomes. Descriptive statistics, paired/unpaired two-tailed t-test, Pearson's or Spearman's correlation were applied as appropriate.
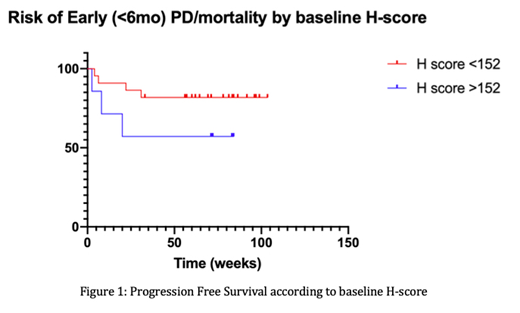
Results: Correlative BM biopsies were collected for 29 pts [male = 18, mean age = 65.0 years (range 41.9-83.2), 2 median prior lines of therapy (range 1-3)] at baseline, 21 pts after 6 months of KTd (7 had PD/died prior to cycle 6, 1 came off study due to grade 4 AE after 5 cycles) and 5 pts at time of PD. There was no difference in the number of fields, BM cellularity (%) or number of nucleated cells (NCs) assessed at baseline and 6mo (p=0.927, 0.331 and 0.491 respectively). PC burden (%; mean±SD) reduced significantly following 6mo KTd (28.3±28.1 vs. 2.12±2.37; p=0.0007). The number of plasma cells expressing csGRP78 (% of all NCs) was reduced following 6mo KTd treatment (27.04±26.83 vs. 2.05±2.32; p=0.0007) while the % of CD138-ve BM cells expressing csGRP78 (% of all NCs; mean±SD) increased (62.61±27.55 vs. 87.46±10.11; p=0.0005). Globally, there was a trend for reduced intensity of csGRP78 expression after 6mo KTd (H-score 70.79±62.16 vs. 53.53±51.45; p=0.2073). There was no correlation between baseline BM NC GRP78 H-score and baseline paraprotein level, involved/uninvolved serum free light chain ratio or depth of response to KTd. Pts with early (<6mo) disease progression/mortality (n=7) had a significantly higher baseline H-score (136±78.8 vs. 75.1±65.2; p=0.049). There was a separation of survival curves, but no significant difference regarding risk of early PD/mortality based a baseline H-score >75th percentile of the cohort (>152); p=0.1472 (Figure 1).
Conclusion: Here we demonstrate that cell surface expression of GRP78 is prominent in both the plasma cells and cells of the tumour microenvironment in patients with RRMM and persists in the TME cells in patients on treatment. Early (<6mo) disease progression or mortality is associated with higher baseline intensity of global cell surface GRP78 expression. Additional studies are being performed to evaluate the promise of cell surface GRP78 expression on plasma cells as a potential biomarker of response to therapy.
Harrison:AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: investigator on studies, Research Funding; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen Cilag: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; GSK: Consultancy, Research Funding. Quach:Takeda: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees; GSK: Membership on an entity's Board of Directors or advisory committees; Karyopharm: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal