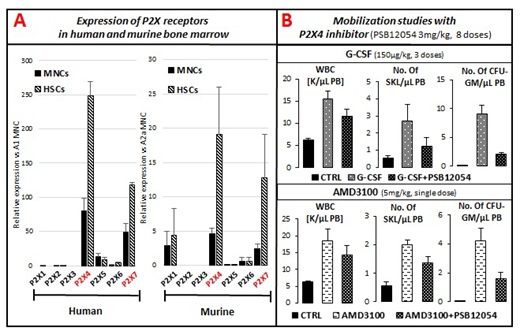
Background. Adenosine triphosphate (ATP) is an important nucleotide involved in intracellular energy transfer, but it is also released from activated cells into the extracellular space as a crucial component of the purinergic signaling network. Purinergic signaling is an ancient form of extracellular signaling that is mediated by ATP and other extracellular nucleotides (EXNs). Purinergic receptors for EXNs are expressed on the surface of all cells in the body; are represented by several families of P1, P2X, and P2Y receptors; and are among the most abundant receptors in living organisms. Of all these receptors, the P2X receptor family is most highly specific for ATP signaling and consists of seven members (P2X1-7). We recently reported that by activating the P2X7 receptor ATP activates the Nlrp3 inflammasome in hematopoietic cells and significantly enhances G-CSF-induced mobilization of hematopoietic stem progenitor cells (HSPCs, Leukemia 2018, 32:1920-1931). Hypothesis. Since P2X7-KO mice still mobilize some HSPCs, we hypothesized that, in addition to the P2X7 receptor, other receptors from this family also play a role in the mobilization process.Materials and Methods. RT-PCR and FACS were employed to phenotype human and murine bone marrow (BM) mononuclear cells (MNCs) as well as sorted HSCs for expression of P2X receptors. Mobilization studies were performed in wild type mice in which the P2X4 receptor was blocked by the specific small-molecule inhibitor PSB12054 and in P2X7-KO animals. Following mobilization, we measured i) the total number of white blood cells (WBCs) and ii) the number of circulating clonogenic colony-forming unit granulocyte/macrophage (CFU-GM) progenitors and Sca-1+c-kit+lineage- (SKL) cells circulating in PB. We also evaluated activation of the Nlrp3 inflammasome as well as other inflammasomes expressed in hematopoietic cells, including Aim2, Nlrp1a, Nlrp1b, and Nlrc4. Results. We found that, of all the receptors of the P2X family, two members, P2X7 and P2X4, are highly expressed on the surface of murine and human MNCs and HSPCs (Figure 1a). Activation of both receptors on the surface of innate immunity CD11b+Gr-1+ cells resulted in activation of the Nlrp3, Nlrp1a, Nlrp1b, and Aim2 inflammasomes. Inhibition of the P2X4 receptor by a specific small-molecule inhibitor decreased G-CSF- and AMD3100-induced mobilization in wild type mice (Figure 1b). Interestingly, we observed differences during AMD3100 mobilization, namely that while P2X7-KO deficiency resulted in decreased G-CSF-induced mobilization and did not affect AMD3100 mobilization efficiency, P2X4 inhibition, by contrast, led to a profound decrease in both G-CSF- and AMD3100-induced mobilization efficiency. Conclusions. Our results further support an important role for purinergic signaling in the mobilization of HSPCs. We also demonstrated that P2X7 and P2X4 receptors, which are highly expressed on human and murine hematopoietic cells, are involved in G-CSF-induced mobilization by activating the Nlrp3, Nlrp1a, Nlrp1b and Aim2 inflammasomes. By contrast, AMD3100-induced mobilization requires expression of P2X4 but not P2X7 receptors, which implies the involvement of different mechanisms and indicates that P2X7 deficiency can be compensated by the P2X4 receptor. Currently, we are performing RNASeq studies to identify differences in the G-CSF- and AMD3100-induced pathways in the mobilization process. These investigations will shed more light on the molecular pathways involved in the egress of HSPCs from BM into PB and will help to design better mobilization protocols in cases of poor mobilizers.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal