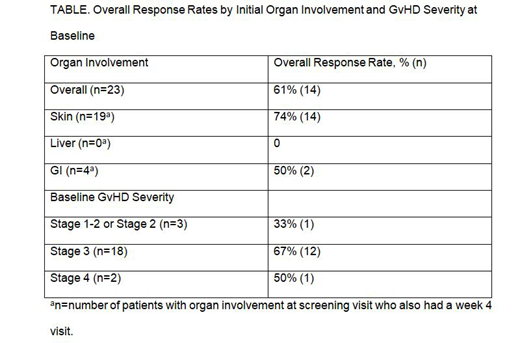
Background: Steroid-refractory acute graft-versus-host disease (SR-aGvHD) is a major cause of morbidity and mortality after allogeneic hematopoietic stem cell transplant in pediatric patients. Second-line therapies cause further immunosuppression, leaving patients vulnerable to life-threatening infections. Extracorporeal photopheresis (ECP) with methoxsalen (Uvadex®) sterile solution, in which apheresed leukocytes are photosensitized, exposed to ultraviolet A radiation ex vivo, and reinfused, is used to treat aGvHD because it has a low risk of infection, unlike systemic immunosuppressants. Safe use of ECP in children is not yet established. Aims: To report interim 4-week data from a 12-week phase 3 study evaluating the efficacy and safety of ECP using the photosensitizing agent methoxsalen with the Therakos® Cellex® Photopheresis System (Cellex; Mallinckrodt Pharmaceuticals, Bedminster, NJ) in pediatric/young adult patients with SR-aGvHD (NCT02524847). Methods: In this single-arm, open-label study, patients (aged 1-21 years; SR-aGvHD International Bone Marrow Transplant Registry [IBMTR] grade B-D) received ECP 3 times/wk for 4 weeks, followed by 2 times/wk for 8 weeks. Steroids were tapered according to physician discretion if SR-aGvHD responded to ECP. The primary endpoint was proportion of patients with an overall response (OR), defined using the Modified IBMTR Severity Index as complete response (CR; complete resolution of aGvHD in all evaluable organs, without next-line systemic treatment) or partial response (PR; improvement of 1 stage in ≥1 aGvHD target organ without progression in others or addition of next-line systemic treatment), after 4 weeks. The primary endpoint was considered significant if P<0.005 for CR+PR vs the null hypothesis (10% response rate), which corresponded to CR+PR >48%. Secondary outcome measures included safety, duration of response, steroid-sparing effect, and organ-specific response. Results: Of 25 patients enrolled, 25 were evaluable for safety (patients who received ≥1 ECP treatment) and 23 were evaluable for efficacy. Patients' mean age was 9.2 years, 44% were female, mean body mass index was 18.3 kg/m2, and the majority (n=18) had Grade 3 aGvHD. Twelve patients (48%) completed all study visits; 5 withdrew early (2 deaths, 1 CR; 3 withdrew due to inadequate efficacy, 2 of whom started second-line therapy, and 3 no longer required treatment) A median of 27 ECP treatments were administered (range 3-28). OR was 61% (14/23 evaluable patients); there were 3 CRs (13%) and 11 PRs (48%; P<0.00001 vs null hypothesis; 95% CI: 36.6, 77.9). OR based on initial organ involvement and overall severity is in the Table. Of the 14 patients who responded by 4 weeks, 9 (64%) and 8 (57%) maintained response at 8 and 12 weeks, respectively. Time to disease progression was a median of 89 days (95% CI: 44.0, 104.0) after enrollment. Median duration of first response was 22.0 days (95% CI: 8.0, 49.0). Among 22 patients evaluable for steroid dose, 9 (41%) had ≥50% reduction in dose by week 4. 103 AEs (11 serious), including 2 deaths (cerebral aspergillosis; disease progression leading to respiratory failure), occurred in 19 patients. Of these, 99 (96.1%), including 2 on-study deaths, were considered unrelated to study treatment, and 4 (nausea, hypocalcemia, hypertension, anemia) possibly related. Conclusions: After 4 weeks of ECP using methoxsalen sterile solution and the Therakos Cellex Photopheresis System, the OR rate was 61%. These encouraging data warrant further investigation in pediatric/young adult patients with SR-aGvHD.
Kitko:Mallinckrodt: Honoraria; Novartis: Consultancy, Honoraria. Boodée:Mallinckrodt Pharmaceuticals: Employment.
UVADEXÃ'® (methoxsalen) Sterile Solution is indicated for extracorporeal administration with the THERAKOSÃ'® UVAR XTSÃ'® or THERAKOSÃ'® CELLEXÃ'® Photopheresis System in the palliative treatment of the skin manifestations of Cutaneous T-Cell Lymphoma (CTCL) that is unresponsive to other forms of treatment. Extracorporeal photopheresis (ECP) with methoxsalen (UvadexÃ'®) sterile solution is used to treat acute graft-versus-host disease (aGvHD), but there are few clinical trials in pediatric patients. This abstract reports on the interim results of a phase 3 clinical trial of ECP with methoxsalen used with the TherakosÃ'® CellexÃ'® Photopheresis System in pediatric/young adult patients with steroid refractory aGvHD.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal