Circulating NK cells are the effector arm of the innate immune system. As such, they recognize transformed cells as "non-self" and kill them. Numerous ex vivo studies have demonstrated the ability of NK cells to kill allogeneic leukemia cells. Based on these in vitro studies, several past and ongoing clinical trials have shown allogenic NK cells have a strong anti-leukemia effect. However, the use of NK cells as an "off the shelf" product is limited since they, like most cellular products, induce an allo-reactive immunogenic response which limits efficacy and increases toxicity.
Exosomes are nano scaled extracellular vesicles that are released by various types of cells including NK cells. Since the exosomal cargo reflects, in part, the molecular makeup of its cell of origin, we hypothesized that NK-derived exosomes maintain the anti-leukemia effect of their cell of origin. To test this hypothesis, we exposed leukemia cells to NK-derived exosomes and tested their ability to eliminate them.
As a source for NK-derived exosomes we used the NK-92MI cell line. This is a genetically altered cell line which constitutively expresses IL-2 that augments the cytotoxic activity of the cells and is routinely used in clinical trials. We cultured these cells in exosome free medium for 48 hours and extracted the exosomes by ultracentrifugation. Nanoparticle analyzing system showed that the pellet was enriched with the typical ~100nm sized vesicles and these particles were visualized by electron microscopy. Western immunoblotting confirmed that these particles express CD63, a known exosomal biomarker. Subsequently, we subjected CML K562, ALL Jurkat and AML HL-60 to NK-92MI-Exo stained with PKH-26 and showed by flow cytometry that these cells uptake the exosomes in a dose- and time- dependent manner. As controls, we used exosomes derived from the human embryonic kidney 293 (HEK-293) cell line.
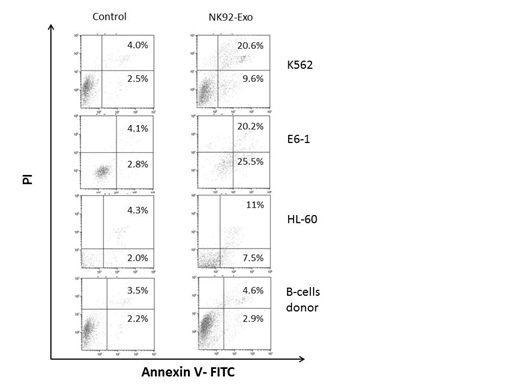
LDH release assay showed a marked increase in LDH activity in NK-92MI-Exo exposed leukemia cells but not in HEK-293-Exo exposed leukemia cells across all cell lines tested. Similarly, flow cytometry of leukemia cells double stained for annexin/PI showed that the rate of apoptosis was markedly increased in NK-92MI-Exo exposed leukemia cells but not in HEK-293-Exo exposed leukemia cells. Together, these assays indicate that NK-92MI-Exo are cytotoxic to various leukemia cell lines and that this effect is specific to NK-derived exosomes. Encouraged by our preliminary results, we harvested leukemia cells from 4 patients with acute myeloid leukemia (AML), 4 patients with acute lymphoblastic leukemia (ALL), 4 patients with chronic lymphocytic leukemia (CLL) and normal B-cells from healthy volunteers and exposed them to NK-92MI-Exo. Similar to the cell line results, we found a marked cytotoxic effect to NK-92MI-Exo on these leukemia cells but not on normal B-cells from healthy individuals whereas HEK-293-Exo had only a minimal or no effect on primary leukemia cells. Clonal assay on peripheral blood sample from 2 CML patients showed that the number of colony forming unit -granulocyte, erythrocyte, megakaryocyte monocyte (CFU-GEMM) is significantly reduced following exposure to NK-92MI-Exo, suggesting that these exosomes target leukemia progenitor cells. Proteomics analysis of NK-92MI-Exo revealed that similar to NK cytotoxic granules, the exosomal cargo include granozymes known to induce apoptosis of target cells but unlike NK cytotoxic granules the exosomal cargo does not include perphorines, known to perforate target cell membrane.
Taken together our data suggests that NK-derived exosomes have a potent cytotoxic effect across a wide range of leukemia cells. This effect is specific to NK-exosomes. Furthermore, NK-derived exosomes preferentially kill leukemia cells but not normal cells. We also show that these exosomes are toxic to leukemia progenitor cells. Proteomics analysis suggested that NK-cytotoxic granules and exosomes use different strategies to enter target-cells. Whether NK-derived exosomes may become a-cellular therapeutic strategy to combat leukemia remains to be determined.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal