Background: Several novel therapies for multiple myeloma (MM) have been approved in recent years. While these therapies have demonstrated improvement in real-world tumor responses and progression-free survival, the cost burden of late-line relapsed refractory MM is not well understood. The objective of this retrospective cohort analysis of US claims data was to examine total, MM-related, and adverse event (AE)-related healthcare costs for patients with MM at different lines of treatment (LOTs), with a focus on patients at third (3L) or later (4L+) LOT.
Methods: The study identified an incident cohort of treatment-naïve patients with MM aged ≥18 years from the PharMetrics Plus (IQVIA™, Durham, NC) database of US commercial claims and included patients had a first diagnosis of MM (Index date) between January 1, 2012 and December 31, 2016 and received ≥1 MM therapy during the ≥1-year follow-up period. Endpoints included all-cause and MM-related costs per patient per month (PPPM), common AEs, AE-related costs per episode, and proportion of patients requiring AE-related hospitalization. Descriptive analyses were conducted based on LOT (first-line [1L], second-line [2L], 3L and 4L+) which was defined using all MM drugs of interest observed within 28 days of use of the first MM drug, whereas end of a LOT was identified using a 90-day gap in the therapy regimen or use of a new agent outside the 28-day window. All-cause and MM-related costs were compared between patients indexed from 2012-2014 and 2015-2017 (ranges based on the availability of new MM drugs) using a univariate generalized model with log link and gamma distribution.
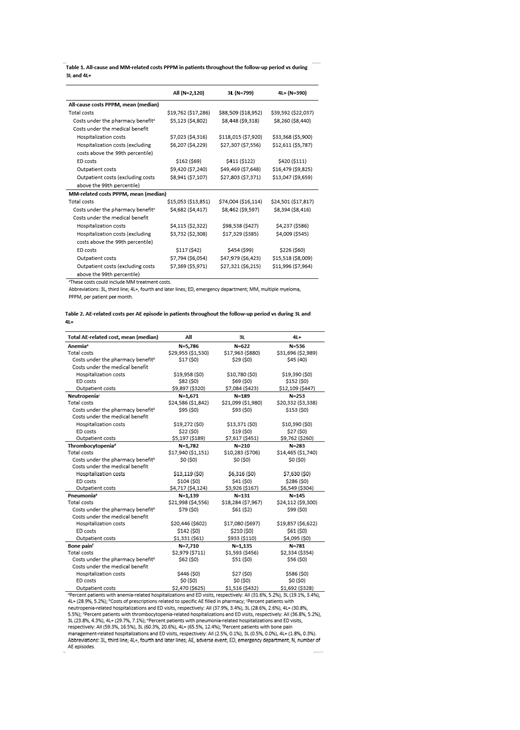
Results: Of the 2,120 patients in the incident cohort, 799 (37.7%) and 390 (18.4%) patients received 3L and 4L+ treatments, respectively. Mean (standard deviation) age at initial MM diagnosis was 58.9 (8.9) and 58.8 (8.6) years for patients progressing to 3L and 4L+. Patients at 3L or 4L+ had greater use of novel therapies (29.2% and 66.9%, respectively) vs patients at 1L (1.4%) and 2L (12.0%). Mean all-cause and MM-related total costs PPPM were higher in 3L and 4L+ vs the mean costs for the entire cohort (Table 1). Costs were significantly greater in patients indexed later (2015-2017) vs earlier (2012-2014) during the study (before inflation adjustments).
The most frequent AEs after initial MM diagnosis included hematologic AEs (anemia [79.3%], neutropenia [40.9%], and thrombocytopenia [38.2%]), pneumonia (32.6%), and bone pain (72.9%). The median duration of most AEs was short (1.0-5.0 days). The AE-related cost per episode of these AEs was high and increased during later lines of therapy, largely driven by increased inpatient and outpatient costs (Table 2). Costs were highest across lines of therapy for anemia, neutropenia and pneumonia.
Conclusion: Patients in 3L and 4L+ of MM treatment incurred high healthcare costs that followed a typical cost distribution ranging from $18,000 to $22,000 PPPM (median values), with several patients incurring much higher costs that swung mean values substantially upward ($89,000 and $40,000 PPPM, respectively). Costs of managing AEs followed a similar distribution but were somewhat similar across LOTs, with median values ranging from $2,000 to $4,500 per episode and mean values exceeding $30,000 per episode. Additional research should focus on better understanding patients with high cost values, and other costs of managing MM and associated AEs that were not addressed in this analysis.
Acknowledgments: Medical writing assistance was provided by Mary E. Morgan PhD at Fishawack Indicia Ltd UK and funded by GlaxoSmithKline. Programming was provided by Kainan Sun, an employee of IQVIA.
This study (HO-18-18615) was funded by GlaxoSmithKline.
Felber:GlaxoSmithKline: Employment, Equity Ownership. Chen:IQVIA: Employment; GSK: Research Funding. Willson:GSK: Equity Ownership; GSK: Employment. Bell:GlaxoSmithKline: Equity Ownership; GlaxoSmithKline: Employment. Simard:GlaxoSmithKline (former employee): Employment; Medtronic (current employee): Employment. Nunna:GSK: Research Funding; IQVIA: Employment. Dharmani:GSK: Employment. Stirnadel-Farrant:GSK: Employment, Equity Ownership. Wang:GSK: Employment; BMS: Equity Ownership. Bruno:GSK: Employment; BMS: Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal