Introduction: Multiple response criteria have been proposed for the assessment of treatment response in lymphoma patients (pts). The Lugano 2014 criteria have been adopted as the current standard for response assessment in lymphoma (Cheson et al. J Clin Oncol 2014), incorporating 18F fludeoxyglucose (FDG)-positron emission tomography (PET)-computed tomography (CT) into standard staging of FDG-avid lymphomas. For non-FDG avid lymphomas, and when PET is not available, Lugano criteria use bi-dimensional tumor measurements of up to six CT target lesions. The more recently proposed Response Evaluation Criteria in Lymphoma (RECIL) (Younes et al. Annals Oncol 2017) were developed based on the hypothesis that uni-dimensional measurements of up to three target lesions could be used to assess response at a similar level of accuracy to the Lugano criteria. The prognostic value of Lugano 2014 versus RECIL 2017 criteria has not yet been assessed in large clinical trials. We compared the prognostic performance of Lugano and RECIL criteria in pts from the Phase III GOYA study (NCT01287741), which compared the efficacy of obinutuzumab (GA101, G) and rituximab (R) in combination with CHOP (G-CHOP vs R-CHOP) in previously untreated pts with CD20-positive DLBCL.
Methods: In the GOYA study, pts were randomized 1:1 to receive either G-CHOP or R-CHOP (stratification factors: number of planned chemotherapy cycles, International Prognostic Index [IPI], and geographic region). FDG-PET scans were mandatory at sites where a PET scanner was available and were performed at screening and 6-8 weeks after the last study treatment. Response was assessed prospectively based on Cheson 2007 criteria and retrospectively according to standard Lugano 2014 criteria. A retrospective analysis based on RECIL 2017 criteria was also performed. Response categories by RECIL criteria were cross-tabulated against those by Lugano criteria. Estimates of the treatment effect were expressed as hazard ratios (HRs) with 95% confidence intervals (CIs) using stratified log-rank tests. The impact of covariates on PFS and OS were analyzed using a multivariate Cox model. Landmark analyses of PFS and OS according to end of treatment (EoT) complete response (CR)/non-CR status were performed, and the prognostic value of response at EoT was compared for the two methods.
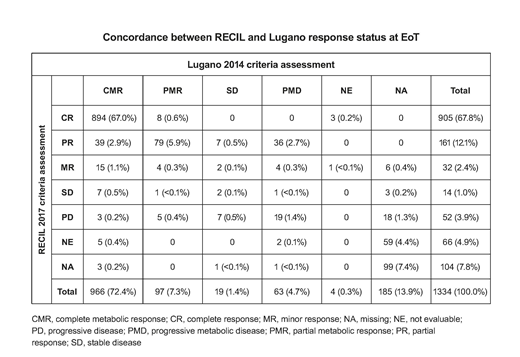
Results: Of the 1418 pts (intention-to-treat [ITT] population) in the GOYA study, 1334 had PET data and were evaluable for this analysis. Good agreement between Lugano and RECIL criteria was observed for EoT CR status, with 894/966 (92.5%) pts with a complete metabolic response (CMR) by Lugano classified as CR by RECIL (Table). However, 40/63 (63.5%) pts with progressive metabolic disease (PMD) at EoT according to Lugano criteria had a partial response (PR) or minor response (MR) by RECIL criteria, showing poor agreement for these variables. Of the 43 pts with PMD by Lugano and non-PD by RECIL at EoT, 15 pts had no PFS event by Lugano as assessed by CT and could be considered false positives. Concordance for PFS by CT was high between the two criteria, with a kappa estimate of 0.77 (95% CI: 0.74, 0.80). EoT CR status by RECIL was highly prognostic for PFS (stratified HR, 0.32; 95% CI: 0.25, 0.43; p<0.0001) and OS (stratified HR, 0.26; 95% CI: 0.19, 0.36; p<0.0001), with a similar prognostic performance as the Lugano criteria (PFS HR, 0.31; 95% CI: 0.23, 0.41; p<0.0001). Multivariate analysis confirmed that EoT CR status by RECIL was prognostic for both PFS (HR, 0.18; 95% CI: 0.15, 0.23; p<0.0001) and OS (HR, 0.23; 95% CI: 0.17, 0.32; p<0.0001), independent of the stratification factors (IPI, number of planned CHOP cycles, geographic region). Assessment with RECIL criteria provided similar PFS results to GOYA for the comparison of G- vs R-CHOP (RECIL: HR, 0.95; 95% CI: 0.78, 1.17; p=0.64; GOYA: HR, 0.94; 95% CI: 0.78, 1.12; p=0.47).
Conclusions: EoT CR status according to RECIL 2017 criteria showed a high concordance with CMR status by Lugano 2014 criteria and was highly prognostic for PFS and OS in previously untreated DLBCL; however, discordance was seen for the identification of PD by RECIL compared with Lugano criteria. PFS as assessed by CT by RECIL, based on uni-dimensional size measurements of up to three target lesions, showed high concordance with PFS by Lugano criteria, based on bi-dimensional size measurements of up to six target lesions.
Kostakoglu:F. Hoffman-La Roche: Consultancy; Genentech: Consultancy. Martelli:F. Hoffman-La Roche, Celgene, Janssen, Sandoz, Novartis, Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees; Servier: Honoraria; Servier: Honoraria; F. Hoffman-La Roche, Celgene, Janssen, Sandoz, Novartis, Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees. Sehn:Astra Zeneca: Consultancy, Honoraria; Acerta: Consultancy, Honoraria; Merck: Consultancy, Honoraria; Lundbeck: Consultancy, Honoraria; F. Hoffmann-La Roche/Genentech: Consultancy, Honoraria, Research Funding; TG Therapeutics: Consultancy, Honoraria; Janssen-Ortho: Consultancy, Honoraria; Morphosys: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; Morphosys: Consultancy, Honoraria; Seattle Genetics: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Karyopharm: Consultancy, Honoraria; Merck: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; TG Therapeutics: Consultancy, Honoraria; Verastem: Consultancy, Honoraria; Janssen-Ortho: Honoraria; Lundbeck: Consultancy, Honoraria; Kite Pharma: Consultancy, Honoraria; Kite Pharma: Consultancy, Honoraria; Acerta: Consultancy, Honoraria; F. Hoffmann-La Roche/Genentech: Consultancy, Honoraria, Research Funding; TEVA Pharmaceuticals Industries: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Seattle Genetics: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; TEVA Pharmaceuticals Industries: Consultancy, Honoraria; Astra Zeneca: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; Apobiologix: Consultancy, Honoraria. Trněný:Amgen: Consultancy, Honoraria; Gilead Sciences: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Incyte: Consultancy, Honoraria; MorphoSys: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; Bristol-Myers Squibb: Consultancy, Honoraria; F. Hoffmann-La Roche: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Celgene: Consultancy. Vitolo:Abbvie: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; F. Hoffmann-La Roche: Speakers Bureau; Celgene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Juno Therapeutics: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Kite: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Knapp:F. Hoffmann-La Roche Ltd: Employment. Mattiello:F. Hoffmann-La Roche Ltd: Employment. Nielsen:F. Hoffmann-La Roche Ltd: Employment, Equity Ownership. Sahin:F. Hoffmann-La Roche Ltd: Employment, Equity Ownership. Sellam:Roche: Employment, Equity Ownership. Ward:Hoffmann La Roche: Employment, Equity Ownership. Younes:Roche: Consultancy, Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Curis: Honoraria, Research Funding; Merck: Honoraria, Research Funding; Abbvie: Honoraria; Takeda: Honoraria; Pharmacyclics: Research Funding; AstraZeneca: Research Funding; Genentech: Research Funding; Biopath: Consultancy; Xynomics: Consultancy; Epizyme: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; HCM: Consultancy; BMS: Research Funding; Syndax: Research Funding.
GAZYVA (obinutuzumab) is a CD20-directed cytolytic antibody and is indicated for the following: in combination with chlorambucil, for the treatment of patients with previously untreated CLL; in combination with bendamustine followed by GAZYVA monotherapy, for the treatment of patients with FL who relapsed after, or are refractory to, a rituximab-containing regimen; in combination with chemotherapy followed by GAZYVA monotherapy in patients achieving at least a partial remission, for the treatment of adult patients with previously untreated stage II bulky, III or IV FL.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal