Introduction:
Fostamatinib, a spleen tyrosine kinase (Syk) inhibitor has been studied in clinical trials of chronic immunologic conditions such as Rheumatoid Arthritis (RA), chronic immune thrombocytopenic purpura (ITP), IgA nephropathy and certain lymphomas. It has recently been granted FDA approval for the treatment of ITP. Fostamatinib inhibits the Syk pathway which is also involved in platelet activation through collagen receptor and the integrin αIIbβ3, which, in theory, would increases the risk of bleeding. Also, by inhibiting Syk, fostamatinib reduces macrophage phagocytosis and may render them ineffective against certain bacteria, hence increasing the risk of serious infections. We sought to examine the side effect profile of Fostamatinib in published and unpublished studies randomized controlled trials (RCT).
Methods:
A systematic search of scientific databases, major conference abstracts and clinical trial registries was performed. Only Phase 2 and Phase 3 RCTs with a placebo arm were included. For dosing of fostamatinib, we preferentially used the 100mg BID dosing as this is the dose approved by the FDA for ITP and is the dose determined through the large trials in patients with RA, which strikes a balance between benefits and harms. When the 100mg and 150mg dosing were combined (as in the ITP trials), we used data from that arm for the analysis. All major and minor harms specified in the trials were pooled using a random effects model and the risk ratio (RR) and confidence interval (CI) was determined using the Mantel-Haenszel method. An I2 value of less than ≤ 40% was considered minimal heterogeneity.
Results:
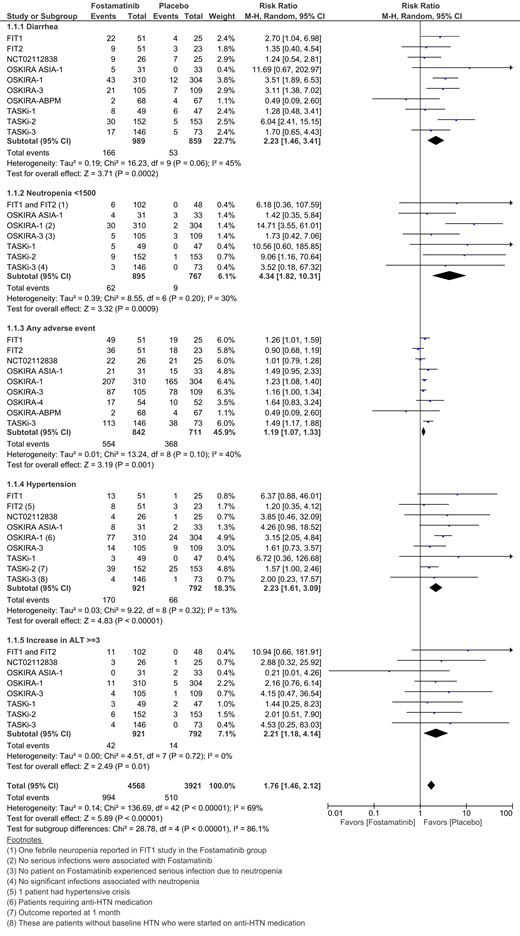
The search found 12 studies involving 1,444 cases and 1,188 controls. Of these, 9 studies examined the use of fostamatinib for RA whereas 2 studies were on ITP, and 1 study was on IgA nephropathy. Commonly encountered side effects of fostamatinib therapy were diarrhea, headache, nausea and hypertension. When compared to placebo, fostamatinib was associated with 19% higher risk of any adverse event (9 studies, RR = 1.19, CI = 1.07 - 1.33, I2 = 40%). Patients who received fostamatinib had a significantly higher risk of developing neutropenia (ANC < 1500/microL) when compared to placebo (8 studies, RR = 4.34, CI = 1.82 - 10.31, I2 = 30%). There was only 1 case of febrile neutropenia in one of the ITP trials. There were no significant differences between the fostamatinib and placebo groups with regard to upper respiratory tract infections (7 studies, RR = 1.43, CI = 0.61 - 3.36, I2 = 49%), urinary tract infections (4 studies, RR = 1.6, CI = 0.78 - 3.28, I2 = 0%) or serious infections (7 studies, RR = 1.18, CI = 0.42 - 3.30, I2 = 0%). However, when compared to placebo, there was a 2.23 times higher risk of developing diarrhea (10 studies, CI = 1.46 - 3.41, I2 = 45%) and hypertension (9 studies, CI = 1.61 - 3.09, I2 = 13%) in the fostamatinib group. Most patients had hypertension at baseline and few needed either medication initiation or adjustment in the fostamatinib cohorts. Fostamatinib also significantly increased liver enzyme (ALT > 3 ULN) when compared to placebo (9 studies, RR = 2.21, CI = 1.18 - 4.14, I2 = 0%). There were higher bleeding events in the fostamatinib group, but there was no significant difference between the treatment and placebo arms (4 studies, RR = 1.06, CI = 0.16 - 6.94, I2 = 45%). There were no significant differences between the treatment and control groups with regard to serious adverse events and mortality. Treatment discontinuation rates due to adverse events were not significantly different between groups.
Conclusions:
Fostamatinib tends to have a relatively benign side effect profile, with few serious side effects. In congruence of the theoretical higher bleeding risk with Syk inhibition, the bleeding events were slightly higher in fostamatinib group, however there was no statistically significant difference between the treatment and the placebo groups. Similarly, the incidence of neutropenia, though higher in the Fostamatinib group, was rarely associated with fever (1 event among all 12 trials). The incidence of serious infections did not differ significantly between groups. Gastrointestinal and cardiac side effects were transient and did not lead to significantly more treatment discontinuations when compared to placebo. Larger longitudinal studies are needed to better examine the long-term side effects associated with Fostamatinib.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal