BACKGROUND
Selection biases can impair the generalizability of clinical trials. Studies investigating aggressive diseases such as Diffuse Large B-cell Lymphoma (DLBCL) can be particularly affected by such biases since clinical urgency and need for therapy may not allow the requisite extensive screening and consent processes for trials. Diagnosis-to-Treatment Interval (DTI) has recently been proposed as a novel metric to capture this phenomenon (Maurer et al, JCO, 2018), and short DTI is associated with both adverse clinical factors and adverse clinical outcomes. Intriguingly, DTI was independent of clinical risk factors like the International Prognostic Index (IPI) suggesting that widely applied prognostic scores do not adequately reflect risk factors considered for clinical decision making. In this study, we aim to assess whether pretreatment levels of circulating tumor DNA (ctDNA) are associated with shorter DTI and may constitute an objective measure of clinical urgency.
METHODS
We quantified pretreatment ctDNA levels in plasma samples from 178 patients treated in 5 US and European centers for large cell lymphoma (DLBCL, Follicular lymphoma grade 3b, or High-grade-B-cell-lymphoma) using Cancer Personalized Profiling by Deep Sequencing (CAPP-Seq) as previously described (Kurtz, JCO 2018; Scherer, STM 2016). Pretreatment ctDNA levels were correlated with DTI, clinical factors and treatment outcome.
RESULTS
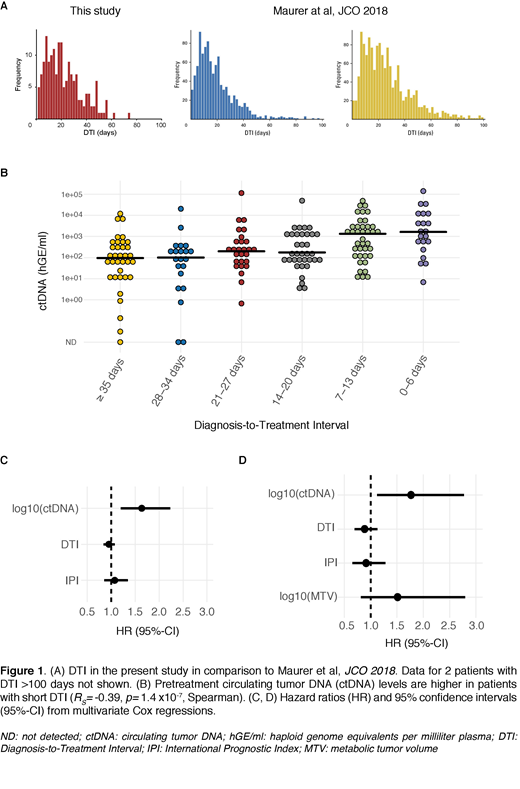
Pretreatment ctDNA was detectable in 175/178 cases. Median number of single nucleotide variants (SNV) detected per patient was 129 (range 0-628). Pretreatment ctDNA levels ranged from 0 - 1.4 x105 haploid genome equivalents per milliliter of plasma (hGE/ml, median 239). Median DTI was 19 days (range 0-141, Figure 1A) and was similar in distribution to 2 previously described cohorts from the US and Europe (Maurer et al, JCO 2018). Shorter DTI was associated with higher ctDNA levels (RS=-0.39, P= 1.4 x10-7, Figure 1B). Patients with longer DTI had improved Event-Free Survival (EFS, Hazard Ratio (HR) for DTI: 0.9/week, P= 0.03). However, this association was lost when adjusting for pretreatment ctDNA levels (HR for DTI: 0.95/week, P= 0.39; HR for log10(ctDNA): 1.7, P= 5.8 x10-5). In a multivariate analysis including DTI, ctDNA and IPI, only ctDNA levels were significantly associated with EFS (HR for log10(ctDNA): 1.6, P= 0.002, n=178, Figure 1C). Pretreatment ctDNA levels remained the only prognostic factor for EFS in a second multivariate analysis also considering pretreatment metabolic tumor volume (MTV, HR for log10(ctDNA): 1.8, P= 0.01, n=93, Figure 1D).
DISCUSSION
Shorter DTI is associated with higher pretreatment ctDNA levels in patients with aggressive B-cell lymphomas. When comparing to established factors (DTI, IPI, MTV), pretreatment ctDNA levels appear to best predict clinical outcomes. This suggests that quantification of ctDNA better reflects disease burden and treatment urgency than existing clinical biomarkers. Pretreatment ctDNA level may therefore be a valuable metric for disease aggressiveness of patients included in clinical trials, and may help identify studies suffering from selection bias. This may be particularly useful for noncontrolled Phase I/II single arm trials, but also for stratification in randomized trials.
Kurtz:Roche: Consultancy. Dührsen:Alexion: Honoraria; Novartis: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Janssen: Honoraria; Takeda: Consultancy, Honoraria; Celgene: Research Funding; CPT: Consultancy, Honoraria; Amgen: Consultancy, Honoraria, Research Funding; Teva: Honoraria; Roche: Honoraria, Research Funding. Hüttmann:Takeda: Honoraria; Gilead: Honoraria; University Hospital Essen: Employment. Westin:Juno: Other: Advisory Board; Novartis: Other: Advisory Board, Research Funding; Janssen: Other: Advisory Board, Research Funding; Kite: Other: Advisory Board, Research Funding; Curis: Other: Advisory Board, Research Funding; Celgene: Other: Advisory Board, Research Funding; 47 Inc: Research Funding; Unum: Research Funding; MorphoSys: Other: Advisory Board; Genentech: Other: Advisory Board, Research Funding. Gaidano:AbbVie: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Astra-Zeneca: Consultancy, Honoraria; Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sunesys: Consultancy, Honoraria. Rossi:Abbvie: Honoraria, Other: Scientific advisory board; Janseen: Honoraria, Other: Scientific advisory board; Roche: Honoraria, Other: Scientific advisory board; Astra Zeneca: Honoraria, Other: Scientific advisory board; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Diehn:Novartis: Consultancy; BioNTech: Consultancy; AstraZeneca: Consultancy; Quanticell: Consultancy; Roche: Consultancy. Alizadeh:Pfizer: Research Funding; Chugai: Consultancy; Celgene: Consultancy; Gilead: Consultancy; Pharmacyclics: Consultancy; Janssen: Consultancy; Genentech: Consultancy; Roche: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal