Acquired hemophilia A (AHA) is a rare autoimmune disease caused by circulating autoantibodies inhibiting coagulation factor VIII (FVIII), leading to a clinically significant bleeding diathesis. While in half of AHA cases, no underlying cause is identified, in the other half an association with autoimmune diseases, cancer, the use of certain drugs, pregnancy or the post-partum period is found.
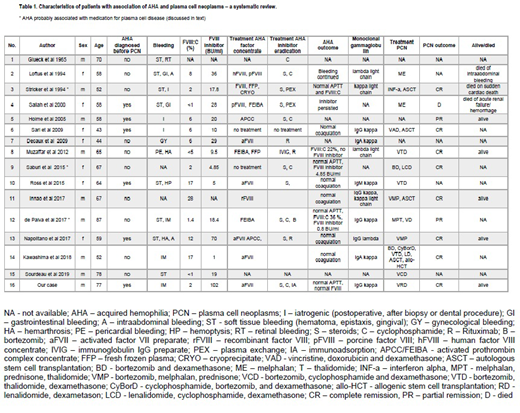
We present a case with severe bleeding in which AHA was diagnosed and subsequently a smoldering myeloma was found as underlying disease (case 16, Table 1). We conducted a systematic review of the literature in PubMed looking for the association of AHA and plasma cell neoplasm (PCN) (key words used: hemophilia, inhibitor, factor VIII, myeloma, plasma cell disorder or neoplasms, smoldering myeloma, MGUS, monoclonal gammopathy, paraprotein) and identified 15 additional cases. Description of the cases, sequence of occurrence of both diseases, their treatment, evolution and outcome are here analyzed (Table 1).
We found that patients having AHA and PCN were more often males (9/16, 56%), had a median age of 61.5 (range 43-87) years at AHA diagnosis. The most common pattern of bleeding was a soft tissue bleeding. In two cases, AHA was diagnosed after excessive post-operative bleeding, in one patient following a life threatening pericardial bleeding and a hemarthrosis. AHA with active bleeding was the presenting sign in 6 cases (38%) (Table 1, cases 4, 5, 6, 10, 13, 16), in search for an underlying diseases, a PCN was diagnosed subsequently. In the other 10 cases, AHA was diagnosed after PCN. In 3 of them, the occurrence of AHA was interpreted to be secondary to multiple myeloma treatment; the implicated drugs were interferon alpha in one and lenalidomide in two cases. Information about the type of monoclonal gammopathy was available in 11 cases; no particular type of paraprotein or clonal light-chain was discernable.
Factor concentrates were used in 12 cases to treat relevant bleedings. Further treatment of AHA consisted of immunosuppression with steroids (N=4), steroids in combination with cyclophosphamide (N=5), or cyclophosphamide alone (N=1). Rituximab was used in 3 cases. Plasma exchange to remove FVIII antibodies was performed in 2 cases, and immunoadsorption was done in our patient. Different therapy regimens were used to treat the underlying PCN over the years (Table 1). At the time of reporting 13 patients were alive, while 2 of 3 patients who died, died of bleeding complications. Information on outcome of AHA and/or PCN was available for 9 of 13 survivors: 6 had normal coagulation tests, while FVIII activity was mildly reduced in two. PCN was in complete and partial remission in 7 and 2 cases, respectively (table 1).
Management of AHA is based on four pillars: avoidance of procedures that may have induced bleedings, control of bleeding, inhibitor eradication and treatment of the underlying disease. Our case, together with those described in the literature, emphasizes the possibility of PCN as an underlying cause of AHA. Accordingly, diagnostics including protein-electrophoresis, immune-fixation and free light chains should be considered in patients with AHA.
In our experience, early intervention with immunoadsorption can be lifesaving in cases with high FVIII inhibitor titers. The occurrence of excessive unexplained bleeding in PCN should raise the suspicion of secondary AHA and trigger the investigation of coagulations tests. Whether PCN treatment alone can control AHA in these cases remains open, 11/16 (69%) of the reported cases received treatment for both diseases.
Jalowiec:Amgen: Other: Travel grant; Pfizer: Other: Travel grant; Novartis: Other: Travel grant. Andres:AbbVie: Membership on an entity's Board of Directors or advisory committees; Janssen-Cilag: Membership on an entity's Board of Directors or advisory committees; Roche: Other: Travel support ; Gilead: Other: Travel support ; Celgene: Membership on an entity's Board of Directors or advisory committees; Novartis: Other: Travel support ; Mundipharma: Other: Travel support ; AbbVie: Other: Travel support; Celgene: Other: Travel support . Kremer Hovinga:CSL-Behring: Honoraria, Other: Honoraria go to employer (Insel Gruppe AG, Department of Hematology); Ablynx/Sanofi: Consultancy, Honoraria, Other: Honoraria go to employer (Insel Gruppe AG, Department of Hematology); Roche: Honoraria, Other: Honoraria go to employer (Insel Gruppe AG, Department of Hematology); Shire: Consultancy, Other: Honoraria go to employer (Insel Gruppe AG, Department of Hematology), Research Funding; Siemens: Honoraria, Other: Honoraria go to employer (Insel Gruppe AG, Department of Hematology).
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal