Introduction
Therapy-related myeloid neoplasms (t-MN) in the revised 2016 World Health Organization classification include cases of acute myeloid leukemia (t-AML), myelodysplastic syndromes (t-MDS, and myelodysplastic/myeloproliferative neoplasms (t-MDS/MPN) in patients exposed to cytotoxic or radiation therapy for an unrelated neoplastic or non-neoplastic disorder (Arber DA,Blood. 2016). It constitutes ~7% of acute myeloid leukaemia (Hulegardh E, 2015).
This is a single center retrospective study aimed to assess the clinicopathologic and genetic characteristics and survival factors for patients with t-MN diagnosed in National Center for Cancer Care and Research, Hamad Medical Corporation over the time period between 2012-2018.
The studied parameters included demographic data, type of original disease, cytotoxic agent, the latency period for developing t-MN, pathologic characteristics of t-MN, TP 53 expression by immunohistochemistry (IHC), cytogenetics and molecular genetic findings. The estimated overall survival is calculated using the Kaplan-Meier method.
Patients and Method:
The inclusion criteria are myeloid neoplasms in patients with a prior history of exposure to the alkylating agent, Topoisomerase II inhibitor, antimetabolites, radiotherapy (including brachytherapy), radioactive iodine, regardless of latency period. A total of 20 patients have met the diagnostic criteria of t-MN out of 232 total patients diagnosed as AML with an overall incidence of (8.6%).
The median age of the cohort was 49 years (16-72 years). Females are predominant 12 (60%) with almost equal distribution between different age groups (<50 and above 50 years old).
Results:
According to the type of original disease, three groups were identified; solid cancers, lymphoid neoplasms, and autoimmune diseases (AID). 12 patients (60%) were treated for solid cancers with breast cancer being the most encountered. Four patients (20%) had prior lymphoid malignancies (multiple myeloma, chronic lymphocytic leukemia, Burkitt lymphoma, and acute lymphoblastic leukemia). The final group include 4 patients (20%) who had AID including: Chron's disease , ankylosing spondylitis or aplastic anemia.
The mean latency period before developing t-MN was 45.9 months (3.8 years) with a range of [15.8-221.1] months; 11 patients(55%) had a latency of ≤5 years and 9 patients (45%) had a latency period >5 years.
The cytogenetic findings in our cohort include recurrent genetic abnormalities which were detected in 8 patients (40%). 4 patients (20%) had complex karyotype. A normal karyotype was detected in 5 patients (25%). 4 patients showed abnormalities of chromosome 5 and 7.
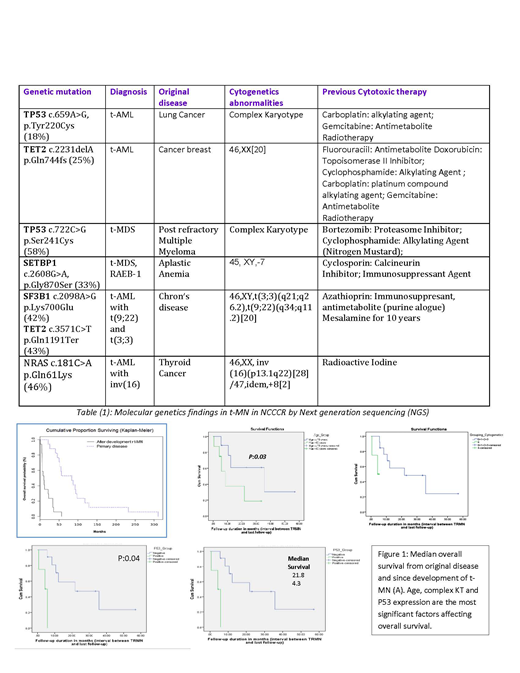
Next-generation sequencing (NGS) had been used to analyze targeted regions in 19 genes recurrently mutated in myeloid neoplasia. Preliminary results are listed in table (1). TP53 gene is a frequently mutated gene in our cohort. Other mutated genes detected with a frequency of >5% include TET2, SETPB1, SF3B, NPM1, IDH-1, and NRAS.
P53 protein expression by IHC was assessed; a cutoff of > 1% p53 strongly positive cells (3+) was used to define p53-positivity. The patients were categorized according to P53 expression into three groups.
Only the group of patients with strong p53 positivity (3+) was well correlated with P53 somatic mutation detected by NGS, and strongly associated with a complex karyotype and poor survival.
Some factors affecting the median overall survival (OS survival) are shown in figure (1).
Conclusion:
Data from our study indicate that TP53 gene is a frequently mutated gene in t-MN. Other mutated genes detected with a frequency of >5% include TET2, SETPB1, SF3B, NPM1, IDH-1, and NRAS. Age, complex KT and P53 expression are significant factors affecting overall survival in t-MN patients.
Patients with t-MN with recurrent genetic abnormalities are specifically enriched in our cohort with higher proportion than published data (~15%) and are not limited to exposure to Topoisomerase II inhibitors. Patients with prior history of autoimmune diseases (in our cohort) showed longer latency period, longer overall survival and negativity for P53 expression.
This is a preliminary data from our cancer center in Qatar. Larger studies in collaboration with other centers in our region regarding the prognostic impact of gene mutations including TP53 are needed before their incorporation into risk stratification.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal