BACKGROUND: Mantle cell lymphoma (MCL) demonstrates a male to female ratio of 4:1. While the pathobiology underlying this gender-distribution imbalance is unknown, anecdotal clinical observations, as well as in vitro evidence of relative androgen receptor (AR) overexpression in MCL cell lines and response to androgen blockade, implicate the AR signaling axis as a potential driver of lymphomagenesis in MCL (Mostaghel et al. Experimental Hematology 2017). Thus, we hypothesized that blocking the AR axis could be a potential treatment strategy for MCL. Here, we report results from our pilot study using the second-generation AR antagonist enzalutamide for treatment of MCL.
METHODS: A single arm pilot study was carried out at the Fred Hutchinson Cancer Research Center/University of Washington/Seattle Cancer Care Alliance. Patients ≥ 18 years, with relapsed/refractory or untreated MCL, metabolically active disease > 1.5 cm in long axis, ECOG ≤ 2, ANC ≥ 1000/mm3 & platelet ≥ 50,000/mm3, and acceptable liver and renal function were eligible. Enzalutamide 160 mg was administered daily until disease progression, intolerance, or decision of patient or physician to stop study drug. The primary endpoint was overall response rate (ORR) including complete response (CR) and partial response (PR). The secondary endpoints were time to treatment failure (TTF), progression free survival (PFS), overall survival (OS), adverse events (AEs) as measured by NCI-CTCAE version 4.0, and disease control rate. Correlative studies included impact of AR expression and changes in sex hormone levels on clinical outcomes.
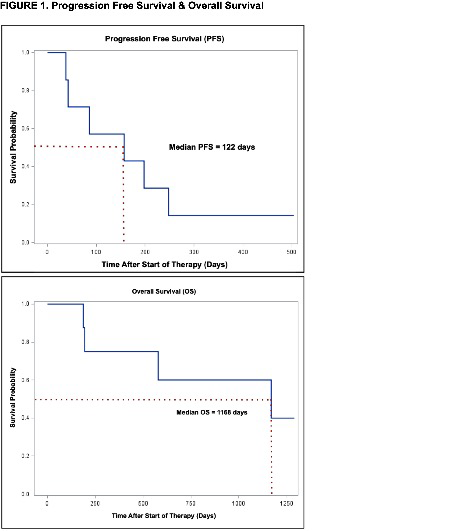
RESULTS: Eight patients were enrolled between September 2015 and January 2018. Median age at enrollment was 60 (range 47-79), six were male, median number of prior treatments was 2.5 (range 0-5), three received prior Bruton's tyrosine kinase (BTK) inhibitor therapy, and four had a prior autologous stem cell transplant. Mantle cell lymphoma international prognostic index (MIPI) scores were low (1), intermediate (4) and high (3). The median Ki-67 was 28% (range < 5 to 60). Patients were treated for a median of 3.5 28-day cycles. Reasons for discontinuation included progressive disease (6) and development of rash at day 10 (1). One patient remains on study and without disease progression at 16 months. Best responses in the six response-evaluable patients included three with stable disease (ranging from 5 to 8 months) and three with progressive disease. Low response rates led to closure of the study. Median PFS and OS were 157 days (range 38 to 248) and 1168 days (range 187 to 1289), respectively (figure 1). All patients had tumor AR expression available for review and stained positive (6 variably positive, 1 uniformly positive, and 1 weakly focally positive). No clinical factor, AR staining nor baseline or changes in hormone levels were correlated with response or PFS, though the single patient with ongoing disease control >15 months is the only evaluable female on study who is receiving enzalutamide as first-line therapy. Two grade 3 (abdominal pain, agitation) and one grade 4 (leukocytosis) AEs were reported.
CONCLUSIONS: Blocking the AR axis with enzalutamide in the treatment of MCL was well tolerated but did not appear to translate into a meaningful ORR despite supportive preclinical data.
This study was approved and funded in part by the National Comprehensive Cancer Network (NCCN) Oncology Research Program from general research support provided by Astellas.
Smith:Merck Sharp & Dohme Corp: Consultancy, Research Funding; Pharmacyclics: Research Funding; Incyte Corporation: Research Funding; Genentech: Research Funding; Denovo Biopharma: Research Funding; Portola Pharmaceuticals: Research Funding; Ayala (spouse): Research Funding; Bristol-Myers Squibb (spouse): Research Funding; Seattle Genetics: Research Funding; Ignyta (spouse): Research Funding; AstraZeneca: Membership on an entity's Board of Directors or advisory committees, Research Funding; Acerta Pharma BV: Research Funding. Till:Mustang Bio: Patents & Royalties, Research Funding. Graf:AstraZeneca: Research Funding; TG Therapeutics: Research Funding; BeiGene: Research Funding. Gopal:Teva, Bristol-Myers Squibb, Merck, Takeda, Seattle Genetics, Pfizer, Janssen, Takeda, and Effector: Research Funding; Seattle Genetics, Pfizer, Janssen, Gilead, Sanofi, Spectrum, Amgen, Aptevo, BRIM bio, Acerta, I-Mab-pharma, Takeda, Compliment, Asana Bio, and Incyte: Honoraria; Seattle Genetics, Pfizer, Janssen, Gilead, Sanofi, Spectrum, Amgen, Aptevo, BRIM bio, Acerta, I-Mab-pharma, Takeda, Compliment, Asana Bio, and Incyte.: Consultancy.
Enzalutamide is FDA approved for metastatic and non-metastatic castration-resistant prostate cancer.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal