AVM0703 is a new treatment option for lymphoma patients that can eliminate or reduce the dose of standard chemotherapy in the overall treatment plan. Radiation and chemotherapy have dramatically improved survival for patients with cancer. However, the American Cancer Society has reported that chemotherapy and radiation increase the chances of secondary cancers. In fact, the report states that chemotherapy is known to be a higher risk factor than radiation in causing leukemia. The need to decrease chemotherapy administration in young adults and pediatric populations becomes even more essential considering the risk for secondary cancers. Therefore, there is an urgent need for new therapies that are as efficacious as chemotherapy and are without the harmful side effects.
AVM0703 is a repurposed small molecule that has significantly extended survival in a mouse model with aggressive B cell lymphoma (A20 mouse model). In addition to being a standalone treatment, AVM0703 can also be administered as a preconditioning agent before CART cell infusion. Two FDA approved breakthrough therapies, Kymriah and Yescarta are not yet available for 33% of patients who are frail and elderly, as they are too weak to undergo the required chemotherapy preconditioning. AVM0703 can have the same preconditioning effect as chemotherapy without the toxic side effects of chemo while sparing red blood cells, stem cells and platelets. AVM0703 demonstrated a significant improvement of the effect of CART cells in a mouse model of melanoma (data not shown). Preconditioning with AVM0703 before CART administration will allow frail and elderly patients access to FDA approved cell therapy treatments.
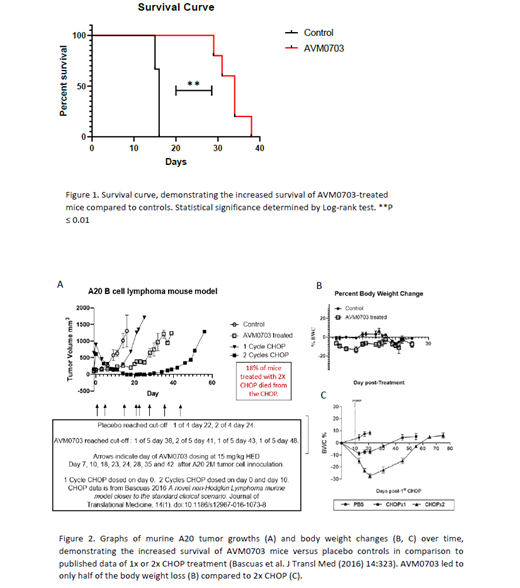
To demonstrate the efficacy of AVM0703 in a lymphoma model, A20 tumor cells were inoculated subcutaneously into the right flank in a cohort of BALB/c mice. When the tumors reached ~ 100mm3, the mice were randomized into two cohorts, control and AVM0703-treated. The control group were vehicle-treated while AVM0703-treated group were administered the active drug on day 7, 10, 17, 23, 24, 28 and 35. Tumor volumes and body weights were measured thrice weekly. Overall the treatment was very well tolerated with no treatment related deaths or toxicity observed. Dramatic body weight loss defined as greater than 20 % decrease from baseline is a frequent concern with chemo treatment, however, it was not observed in any AVM0703-treated mice. The median survival time for the control group was 16 days where as the AVM0703-treated group had a median survival time of 34 days. Therefore, this data suggests that AVM0703 treatment almost doubled the survival time of mice bearing A20 tumors (Figure 1), a fast growing B cell lymphoma model. Most interestingly, when the data was compared to the CHOP regimen, a combination of 4 therapies in the same A20 model, the efficacy results were better than 1 cycle CHOP without the extremely toxic effects observed with CHOP cycles (Figure 2).
As mentioned previously AVM0703 is a repurposed molecule with decades of clinical information available on safety and toxicity, allowing a rapid clinical development program. AVM0703 is a novel high-volume formulation with a target dose that is 25 times what is used currently. It has a unique mechanism of action targeting only cancer cells, lymphocytes and monocytes while sparing platelets, RBCs and stem cells. Due to its unique profile, AVM0703 can replace chemotherapy and reduce the financial and physical toxicities associated with it. AVM0703 presents a new treatment option for lymphoma patients that is safe and effective.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal