Introduction:
Treatment with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) has remained the standard of care as first line treatment for diffuse large B-cell lymphoma (DLBCL) for almost two decades. Treatment outcomes are still suboptimal, as approximately 40% of patients have refractory disease or relapse. Prospective studies recently examined whether the addition of various drugs to R-CHOP (termed R-CHOP+ X) could improve outcomes.
The aim of this study was to compare the efficacy and safety of R-CHOP vs. R-CHOP +X as first line treatment for DLBCL.
Methods:
Systematic review and meta-analysis of randomized controlled trials including patients with DLBCL, comparing first line treatment with R-CHOP vs. R-CHOP+X (i.e. R-CHOP with the addition of another single drug). The Cochrane Library, MEDLINE, conference proceedings and references were searched until June 2019. Two reviewers appraised the quality of trials and extracted data. Primary outcome was overall survival (OS). Secondary outcomes included overall response rate (ORR), complete response (CR) rate, disease control and safety.
Results:
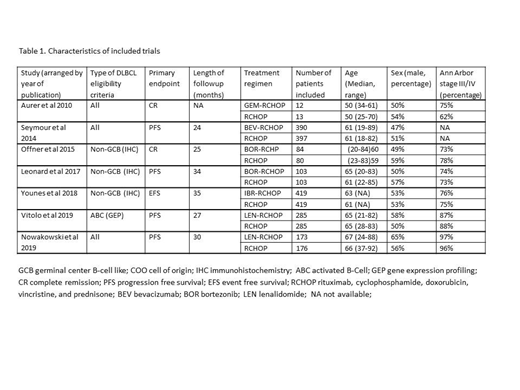
Our search yielded seven trials conducted between the years 2010 and 2019, including 2939 patients (three abstracts, and four published in peer review journals). Median age of patients ranged between 50 to 67 years. The added drug was bortezomib and lenalidomide - each drug in two trials; gemcitabine, bevacizumab and ibrutinib - each drug in one trial. Three trials included all DLBCL patients regardless of cell of origin, whereas four trials included only patients with non-GCB DLBCL. Characteristics of trials included in the meta-analysis are described in Table 1.
Regarding OS, the point of estimate favored improved OS with R-CHOP+X as compared to R-CHOP, yet without statistical significance, HR 0.87, [95% confidence interval (CI) 0.73-1.04, I2=0, 1984 patients, 5 trials].
The ORR and CR rates were similar in the two arms (RR 0.97 [95% CI 0.93-1.02, I2=65%, 2824 patients, 7 trials], and RR 0.99 [95% CI 0.93-1.05, I2=37%, 2826 patients, 7 trials], respectively). However, there was a trend towards improved disease control in the RCHOP+X arm, HR 0.89 [95% CI 0.78-1.01, I2=36].
Regarding safety, three trials (N=1200) reported serious adverse events. There was a significant increase in adverse events in the R-CHOP+X group, RR 1.42 [95% CI 1.24-1.64. I2=55%]. In addition, there was more hematologic toxicity in the R-CHOP+X arm, as reflected by higher rates of grade III/IV thrombocytopenia, anemia and neutropenia.
Conclusions:
The addition of an extra drug to the conventional first line R-CHOP regimen in patients with DLBCL resulted in a trend towards improved disease control compared to standard R-CHOP. However, there was no significant change in response rates or OS, and R-CHOP+ X was associated with an increased risk for serious and grade III/IV hematological adverse events. R-CHOP still remains the "gold standard" of treatment for DLBCL. Further analyses could perhaps reveal subgroups that would benefit from the addition of an additional drug to this regimen.
Gurion:Roche: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal