Introduction: Advanced molecular profiling with next generation sequencing (NGS) has greatly improved the diagnostic and prognostic stratification of hematological disorders. Several driver mutations including TP53, RUNX1, ASXL1, and EZH2 mutations are independent predictors of overall survival (OS) in myelodysplastic syndromes (MDS). Frequent co-mutations of ASXL1, EZH2,RUNX1 and/or TET2 have also been described in the entity. However, studies exploring the clinical significance of co-mutations in these adverse genes are limited. Our study assesses mutation profiles and compares clinical outcomes among MDS patients with EZH2 mutation alone, ASXL1 mutation alone, ASXL1/EZH2 co-mutation, and co-mutated ASXL1/EZH2 with additional mutations.
Materials and Methods: The retrospective study was approved by the institutional review board. We searched our institutional database (>4000 patients) for patients who were diagnosed with MDS and harbored only ASXL1 or EZH2 mutations, only ASXL1/EZH2 co-mutations, and co-mutated ASXL1/EZH2 plus other genes identified by targeted NGS using a myeloid gene panel (up to 54 genes). Patient demographics, diagnosis, cytogenetic abnormalities, and overall survival times were analyzed. Mann-Whitney U Test was used for statistical analysis of OS and a Kaplan Meier survival curve was generated.
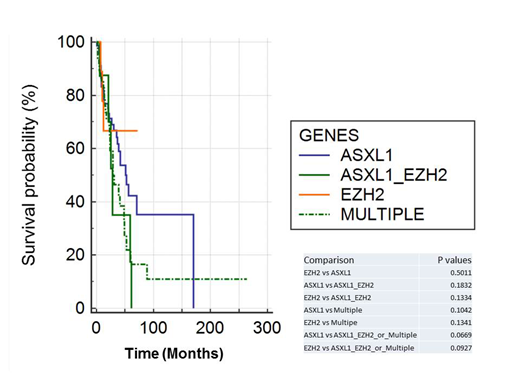
Results: Total 140 patients were retrieved: 71 patients with only ASXL1 mutation, 12 patients with only EZH2 mutation, 8 patients with only ASXL1/EZH2 co-mutation and 49 patients with multiple mutations. As shown in Figure 1, patients with ASXL1/EZH2 co-mutation or multiple mutations show worse OS when compared with patients with EZH2 mutation alone or ASXL1 mutation alone. However, only the differences between either the survival of patients with ASXL1 or EZH2 mutations alone vs multiple mutations are approaching statistical significance (p values = 0.0669 and 0.0927 respectively). Interestingly the patients with ASXL1 mutation alone or multiple mutations appeared more likely to harbor good cytogenetics (70.42% and 65.3%) rather than poor cytogenetics (7% and 2%, respectively). Finally, TP53 mutation appears to be mutually exclusive with ASXL1 or EZH2 mutations, occurring only in 1 patient with multiple mutations.
Conclusion: In our study, MDS patients with ASXL1/EZH2 co-mutations or combined with other mutations appear to show worse prognosis than patients harboring ASXL1 mutation alone or EZH2 mutation alone, though statistical significance was not reached. This could be due to the fact that both ASXL1 and EZH2 confer such an unfavorable prognosis to the patients that additional mutation(s) will not produce any significant clinical impacts. This could also be due to the limited sample size in our study, thus the limited statistical power. A larger cohort study is needed to further explore the prognostic values of co-mutations in this setting. Similar to other reports, TP53 mutation also appears to be mutually exclusive with ASXL1 and/or EZH2; that is of value to further explore the molecular mechanisms.
Sallman:Abbvie: Speakers Bureau; Novartis: Speakers Bureau; Jazz: Research Funding; Incyte: Speakers Bureau; Celyad: Membership on an entity's Board of Directors or advisory committees; Celgene: Research Funding, Speakers Bureau. Komrokji:celgene: Consultancy; pfizer: Consultancy; DSI: Consultancy; Novartis: Speakers Bureau; JAZZ: Speakers Bureau; Incyte: Consultancy; Agios: Consultancy; JAZZ: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal