Background: Patients suffering from chronic lymphocytic leukemia (CLL) are observed and treated intra- as well as extramural during the course of their disease. Generally, hematological centers only see patients who are eligible for active treatment and/or candidates for clinical trials. Thus, a bias between published and real-life patients' data is likely. In our "Hämatologieverbund der WGKK" we take care of CLL patients who are under observation as well as of patients who are in need of treatment. This allows representative analyses of comorbidities and treatment periods during the course of the disease in an in- and outpatient setting. Therefore, a bias in the analyzed population can be excluded. Although new potent oral drugs are now available for the treatment of CLL, still some toxicities are of concern. Disease-related factors as IGHV-mutation and/or TP53-mutation/17p deletion guide specific treatment decisions, but patient-related factors such as age and comorbidities become more and more important. Especially the comorbidities in the usually elderly CLL-population might influence the choice of treatment. Depending on the side effects, caution has to be taken to decide the appropriate individual drug for the patients. Real world data concerning functional status and comorbidities of CLL patients may help to improve treatment results. In a retrospective study 888 patients diagnosed with CLL, having been observed and cared for within the "Hämatologieverbund der WGKK" between 2012 and 2018, were identified and analyzed according to their whole history of disease. Comorbidities were assessed in detail during the whole course of the disease.
Results: At time of diagnosis, the median age was 67.5 years. 96.6% of patients presented with an ECOG-status of 0-1. 89.1% of patients had Binet-stadium A, 8.6% had Binet-stadium B and 2.3% had Binet-stadium C. In 43.2% of patients pathological lymph nodes had been observed and 7.7% of patients reported suffering from constitutional symptoms. 7.5% of patients presented with a del(17p)/TP53 mutation status of ≥10%, while 57.0% of patients were IGHV-mutated. 258 of 888 (29.1%) patients received at least one line of treatment (median 1.7, range 1-6), whereas 71% of patients did not require therapy in the observation period. The most common treatment regimens were: rituximab-bendamustine (BR) (N=135), ibrutinib (N=48), FCR (N=58), chlorambucil combinations (N=39) and venetoclax (N=10). Patients treated with ibrutinib had a comorbidity rate of 41.7% and patients who received BR had a comorbidity rate of 31.9% at time of diagnosis. The mean time from diagnosis to first treatment was 4.0 years (range: 0.0-31.8 years). The median observation time was 6.4 years in both treated and untreated patients (SD 5.6 years, max. 37.0 years). Between the observation period from 2012 to 2018, 17.3% of patients died.
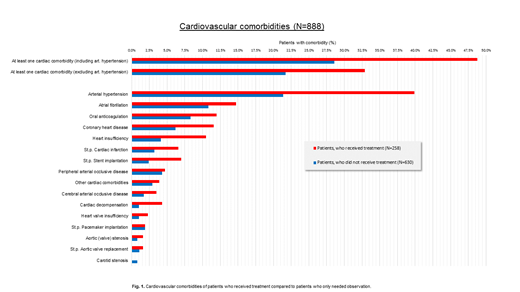
Comorbidities were assessed using the Charlson Comorbidity Index (CCI). 40.0% of patients had at least one comorbidity according to the CCI. Interestingly, patients who received treatment showed the higher percentage of comorbidities (50%) documented during the whole observation period, than patients who never received any treatment (36%). Treated patients showed a rate of comorbidities at time of diagnosis of 30.6%, which increased to 50% during the observation period. Besides the established CCI, we investigated details of cardiovascular comorbidities, including arterial hypertension, in patients who received treatment at time of diagnosis and during the whole observation period (details are shown in Fig. 1). Patients who received ibrutinib in general had more comorbidities compared to all treated patients.
Conclusion: We think that our patient population is representative and the number of patients with p53- and/or IGHV-mutation is similar to previously reported data. It is interesting that the rate of comorbidities in our population group was higher than expected. Especially the rate of cardiovascular comorbidities including arterial hypertension was impressive. Also, patients requiring treatment had more comorbidities than those under surveillance during the observation period. We conclude, that in the era of new drugs with specific toxicities (cardiovascular, renal, pulmonary), awareness, diagnosis and consequent treatment of possibly co-existing comorbidities should be in the central focus in patients suffering from CLL.
Keil:Pfizer: Honoraria; Roche: Honoraria; Takeda: Honoraria, Research Funding; Daiichi Sankyo: Honoraria; AbbVie: Honoraria, Research Funding; Merck: Honoraria, Research Funding; Novartis: Honoraria; Bionorica: Honoraria, Research Funding; Celgene: Honoraria; Janssen: Honoraria, Research Funding. Noesslinger:Janssen: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria; Roche: Consultancy, Honoraria; Gilead: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal