Background: The discovery that gut microbial dysbiosis correlated with prognosis, immune reconstitution and development of graft-versus-host disease (GVHD) in patients undergoing allogeneic stem cell transplantation (allo-SCT) highlights the clinical relevance of the gut microbiome in shaping anti-tumor immune responses. Treatment of allo-SCT patients with antibiotics has recently been associated with increased GVHD mortality (Routy et al. 2017). Based on these studies and the association of distinct gut bacteria with increased efficacy to PD-1 blockade in patients with solid tumors (Derosa et al. 2018), we performed a retrospective analysis to determine if infection treated with antibiotics affected the outcomes of multiple myeloma (MM) patients after autologous SCT (ASCT).
Methods: A list of all MM patients treated at our institution between January 2012 through December 2015 was obtained and 1095 patients were identified. A comprehensive review of the electronic medical record (EMR) of the first 142 who received ASCT was performed. Information was collected from diagnosis to the date of last contact. Baseline characteristics, treatment history, transplant course, antibiotic treatment, and infection severity using common terminology criteria for adverse event (CTCAE) version 4 were reviewed. Prophylactic antibiotics were excluded. Response was measured and defined using the International Myeloma Working Group Criteria. Progression free survival (PFS) and overall survival (OS) were estimated using log rank tests. Cox hazard stepwise regression model examined for multiple factors affecting PFS and OS using the Akaike information criterion.
Results: Of the 142 patients, 93 (65%) were Durie Salmon (DS) III, 20 (14%) were Revised International Staging System (R-ISS) III, 44 (31%) had high-risk cytogenetics, and 76 (54%) were male. The median age at diagnosis was 60. Although there was a similar frequency of DS III (67% vs 61%) and high-risk cytogenetics (35% vs 25%) among patients in the antibiotic and non-antibiotic treated groups, there was an over-representation of R-ISS 3 (19% v 4%) patients in the antibiotic-treated group.
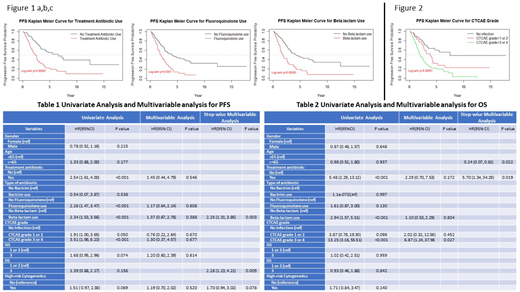
Treatment with antibiotics was associated with decreased median PFS (2.38 vs 6.58 years (yrs), p =0.00003) (Figure 1a) and decreased median OS (7.43 vs 17.39 yrs, p = <0.0001). Fluoroquinolone use compared to no fluoroquinolone use was associated with decreased PFS (1.91 vs 4.09 yrs, p = 0.0001) (Figure 1b) as well as a trend toward decreased OS (7.01 vs 14.23 yrs, p = 0.1). Beta-lactam use compared to no beta-lactam use was associated with decreased PFS (1.74 vs 4.34 yrs, p = <0.0001) (Figure 1c) as well as decreased OS (7.01 vs 17.39 yrs, p = 0.0004). Maximal CTCAE grade of infection throughout the treatment course was a further predictor of decreased PFS-no infection vs CTCAE grade 1/2 vs CTCAE 3/4 (7.49 vs 3.77 vs 1.92 yrs, p = <0.0001) (Figure 2) and OS-no infection vs CTCAE 3/4 (17.39 vs 6.58 yrs, p = <0.0001). (Figure 2).
Multivariate analysis demonstrated progression risk associated with beta-lactam use (HR-2.25, 95% CI, 1.31 - 3.85, p = 0.003), and DSS III (HR-2.28, 95% CI, 1.23 - 4.21, p = 0.009). Multivariate analysis demonstrated increased mortality associated with antibiotic treatment (HR-5.70, 95% CI, 1.34 - 34.29, p = 0.019) and decreased mortality risk with age younger than 65 (HR-0.24, 95% CI, 0.07 - 0.81, p = 0.022). For both PFS and OS, there was no statistical significance demonstrated in multivariate analysis for gender, fluoroquinolone treatment, high risk cytogenetics, R-ISS 3, or CTCAE grade.
Conclusion: In this preliminary study, antibiotic use and infection severity predicted for decreased PFS and OS compared to patients who did not receive antibiotics in MM patients undergoing ASCT. Treatment with at least 2 classes of antibiotics-fluoroquinolones and beta-lactams, predicted for decreased PFS and OS. Multivariate analysis showed increased progression risk with beta lactams and DSS III as well as increased mortality risk with antibiotic treatment and advanced age. The study was limited by its relatively small sample size, retrospective nature, and the high correlation among infection and antibiotic groups that affected the multivariate analysis. Work is underway at our institution to further elucidate the impact of antibiotics on microbial diversity and patient survival.
Goldberg:COTA: Equity Ownership; Bristol-Myers Squibb: Consultancy; Cancer Outcomes Tracking and Analysis (COTA) Inc.: Equity Ownership. Rowley:Allergan: Equity Ownership; Fate Therapeutics: Consultancy. Siegel:Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Bristol-Myers Squibb Company: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Biran:Merck: Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Bristol Meyers Squibb: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal