Background: High-dose chemotherapy (HDCT) followed by autologous transplantation of hematopoietic stem cells (auto-HSCT) is an important component of treatment in multiple myeloma (MM). There is a standard method of controlled cryopreservation of HSC suspension. We found that the storage of native HSC suspension with temperature fluctuations from +3 °C to +5 °C during 72 - 120 hours does not significantly affect the content of CD34+ cells in the product, the index 7AAD- (7-AAD (7-aminoactinomycin - D) is a fluorescent marker that penetrates damaged cell membranes and binds to double-stranded DNA. Through 7AAD does not penetrate intact membranes, so living cells are not stained 7AAD with flow cytometry), and colony-forming ability (CFA) of HSC, as well as the recovery time of hematopoiesis in MM patients after auto-HSCT.
Aim: To evaluate the effectiveness and safety of the method of storage of non-cryopreserved peripheral blood stem cells.
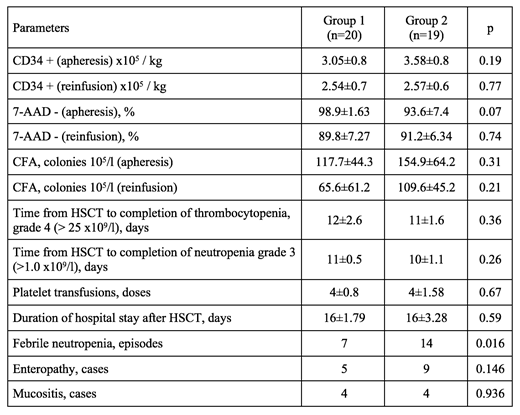
Methods: 39 patients with MM were included in this study(male/female ratio 1.36:1). All the patients get standard immunochemotherapy programs and were in remission at the time of auto-HSCT. Patients were divided into two groups depending on the method of stem cell storage: group 1 - non-cryopreserved (n=20), group 2 - cryopreserved (standard) (n=19). An effectivity and safety were evaluated in such parameters as the number of CD34+ and 7AAD- cells, CFA after apheresis and before reinfusion of HSC. Also, we evaluated the number of platelets concentrate transfusions, the timing of engraftment of granulocytic and megakaryocytic blood sprouts, the length of hospital stays after auto-HSCT.
Results: The results are presented in the comparison table of the evaluated parameters. Our data showed significantly reduce of episodes febrile neutropenia and cases of enteropathy.
Conclusion: Thus, the proposed method of storage of HSC is not inferior to the traditional method with cryopreservation on such parameters as CD34+, 7AAD-, CFA, the number of platelets concentrate transfusions, terms of hematopoiesis restoration, length of hospital stay after HSCT, the number of complications.
Shuvaev:Fusion Pharma: Consultancy; BMS: Consultancy; Novartis: Consultancy; Pfize: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal