Introduction
Multiple sclerosis (MS) is an inflammatory, demyelinating and neurodegenerative disease of the central nervous system (CNS) that causes a whole spectrum of neurological disorders associated with a profound decrease in the quality of life of affected patients. Currently, autologous hematopoietic cell transplantation (ASCT) is a validated therapeutic approach and has been shown to be superior to new immunomodulatory agents. However, the impact of these therapies on the quality of life of patients with MS is unknown.
Objective
Identify the impact on the quality of life in patients with multiple sclerosis after ASCT at our center.
Methods
A quasi-experimental, longitudinal, prospective and single-center study was conducted in which the quality of life was determined in patients with MS before and after ASCT. Patients who could not answer the questionnaire themselves were excluded and incomplete questionnaires were eliminated. The quality of life was determined by applying the MS-QoL 29 instrument which is validated instrument for this pathology (Cronbach 0.88-0.90 and Pearson with high correlation with MS-QoL56). The variables related to the physical and mental components of the instrument as well as demographic characteristics were studied. The statistical analysis of the data included measures of central tendency as well as inferential for the comparison of means and proportions (NC 95%, p <0.05).
Results
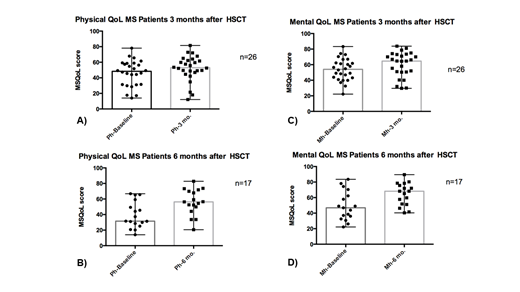
We included 52 patients prospectively from October 2018 to June 2019, 71% of the patients were women and the remaining 29% men. The median of age of the subset is 50 years (Interval 27-65). Of the selected patients, 45% has PPMS, 39% has SPMS and 16% has PPMS. Twenty six patients were followed at 3 months and seventeen were followed 6 months after ASCT. The statistical differences between the quality of life in the patients prior to the ASCT and the follow-up at 3 and 6 months in both the physical and mental components was analyzed. In the physical component the differences at 3 months (A) were significant (p = 0.019, 95% NC) as well as the differences at 6 months (b) after ASCT (p = 0.0024, 95% NC). In the mental component the differences were significant at 3 months (C) (p = 0.0012, NC 95%) as well as the differences at 6 months (D) after ASCT (p = 0.0007, NC 95%).
Conclusions
The study suggests that ASCT is a feasible and safe therapeutic alternative to improve the quality of life in patients with multiple sclerosis.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal